Structure-function correlations in tyrosinases
- PMID: 26104241
- PMCID: PMC4570531
- DOI: 10.1002/pro.2734
Structure-function correlations in tyrosinases
Abstract
Tyrosinases are metalloenzymes belonging to the type-3 copper protein family which contain two copper ions in the active site. They are found in various prokaryotes as well as in plants, fungi, arthropods, and mammals and are responsible for pigmentation, wound healing, radiation protection, and primary immune response. Tyrosinases perform two sequential enzymatic reactions: hydroxylation of monophenols and oxidation of diphenols to form quinones which polymerize spontaneously to melanin. Two other members of this family are catechol oxidases, which are prevalent mainly in plants and perform only the second oxidation step, and hemocyanins, which lack enzymatic activity and are oxygen carriers. In the last decade, several structures of plant and bacterial tyrosinases were determined, some with substrates or inhibitors, highlighting features and residues which are important for copper uptake and catalysis. This review summarizes the updated information on structure-function correlations in tyrosinases along with comparison to other type-3 copper proteins.
Keywords: X-ray structure; catechol oxidase; copper; hemocyanin; type-3 copper proteins; tyrosinase.
© 2015 The Protein Society.
Figures


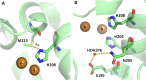

References
-
- Claus H, Decker H. Bacterial tyrosinases. Syst Appl Microbiol. 2006;29:3–14. - PubMed
-
- Faccio G, Kruus K, Saloheimo M, Thöny-Meyer L. Bacterial tyrosinases and their applications. Process Biochem. 2012;47:1749–1760.
-
- Fairhead M, Thony-Meyer L. Bacterial tyrosinases: old enzymes with new relevance to biotechnology. New Biotechnol. 2012;29:183–191. - PubMed
-
- Sanchez-Ferrer A, Rodriguez-Lopez JN, Garcia-Canovas F, Garcia-Carmona F. Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta. 1995;22:1–11. - PubMed
-
- Ramsden CA, Riley PA. Tyrosinase: the four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Biorg Med Chem. 2014;22:2388–2395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

