Factors affecting larval tick feeding success: host, density and time
- PMID: 26104393
- PMCID: PMC4488054
- DOI: 10.1186/s13071-015-0955-6
Factors affecting larval tick feeding success: host, density and time
Abstract
Background: Ectoparasites rely on blood-feeding to sustain activity, support development and produce offspring. Blood-feeding is also a route for transmission of diverse vector-borne pathogens. The likelihood of successfully feeding is thus an important aspect of ectoparasite population dynamics and pathogen transmission. Factors that affect blood-feeding include ectoparasite density, host defenses, and ages of the host and ectoparasite. How these factors interact to affect feeding success is not well understood.
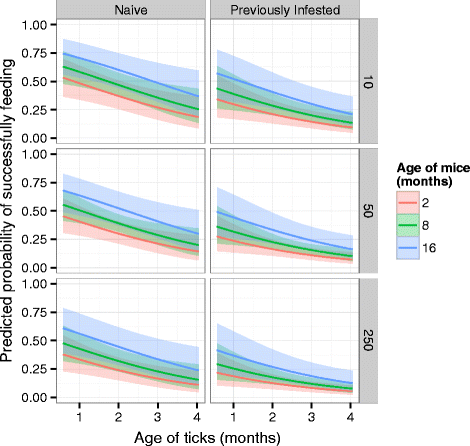
Methods: We monitored blood-feeding success of larval Rocky Mountain wood ticks (RMWTs; Dermacentor andersoni) on deer mice (Peromyscus maniculatus) in several experiments to determine how tick density, host defense, and ages of mice and ticks interact to influence feeding success. In the first experiment, tick-naive deer mice were infested with one of several densities of RMWT larvae, while a second cohort of mice were infested with 50 larvae each. Two weeks after ticks dropped off, mice in the first cohort were re-exposed to 50 larvae each and mice in the second cohort were re-exposed to varying densities of larvae. In the second experiment mice of different ages (45-374 days old) were exposed to 50 larvae each. Two weeks later mice were re-exposed to 50 larvae each. We combined data from these and several similar experiments to test the generality of the patterns we observed. Lastly, we tested whether tick feeding success was consistent on individual mice that were challenged on four occasions.
Results: Mice acquired resistance such that feeding success declined dramatically from the first to the second infestation. Feeding success also declined with tick density and tick age. Mice, however, became more permissive with age. The sizes of these effects were similar and additive. Surprisingly, over successive infestations the relative resistance among mice changed among hosts within a cohort.
Conclusions: We predict that larval blood-feeding success, and thus development to the nymph stage, will change due to variation in tick age and density, as well as the age and history of the host. Incorporating these biotic factors into modeling of tick population dynamics may improve predictions of tick-borne pathogen transmission.
Figures



References
-
- Sonenshine DE. Biology of Ticks, Volume 1. New York: Oxford University Press; 1991.
-
- Lehane M. The Biology of Blood-sucking Insects. 2. New York: Cambridge University Press; 2005. Managing the blood meal; pp. 84–115.
-
- Scott TW, Naksathit A, Day JF, Kittayapong P, Edman JD. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am J Trop Med Hyg. 1997;57:235–239. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

