Different tenogenic differentiation capacities of different mesenchymal stem cells in the presence of BMP-12
- PMID: 26104414
- PMCID: PMC4479325
- DOI: 10.1186/s12967-015-0560-7
Different tenogenic differentiation capacities of different mesenchymal stem cells in the presence of BMP-12
Abstract
Background: Mesenchymal stem cells (MSCs) are regarded as a promising cell-based therapeutic tool for tendon repair. This study aimed to compare the different tenogenic differentiation capacities of the three types of MSCs in the presence of bone morphogenic protein 12 (BMP-12).
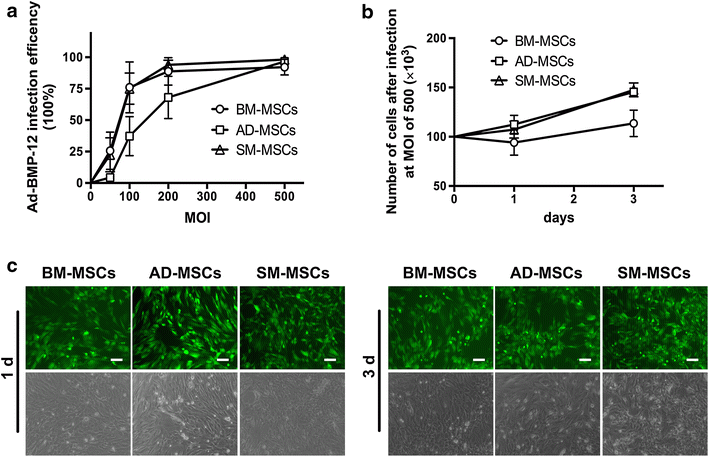
Methods: MSCs were isolated from rat bone marrow (BM), inguinal adipose tissue (AD), and synovium (SM) from the knee joint. MSCs were characterized by morphology, proliferation, trilineage differentiation, and surface marker analysis. Tenogenic differentiation potential was initially assessed using real-time polymerase chain reaction, Western blot, and enzyme-linked immunosorbent assay in vitro. Histological assessments were also performed after subcutaneous implantation of BMP-12 recombinant adenovirus-infected MSCs in nude mice in vivo.
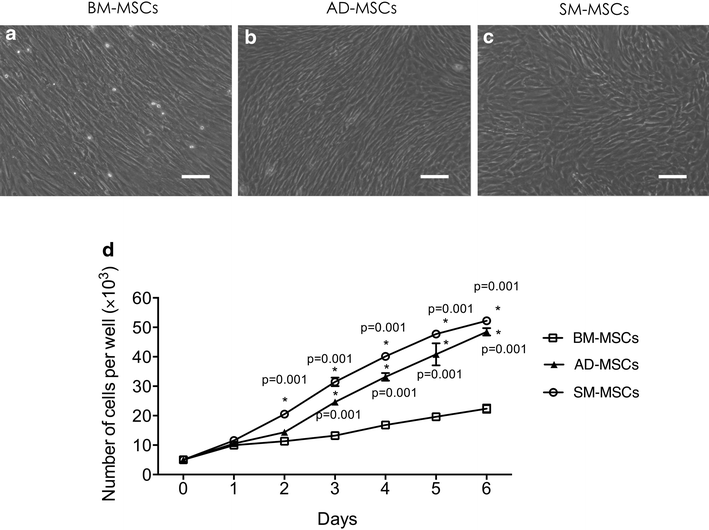
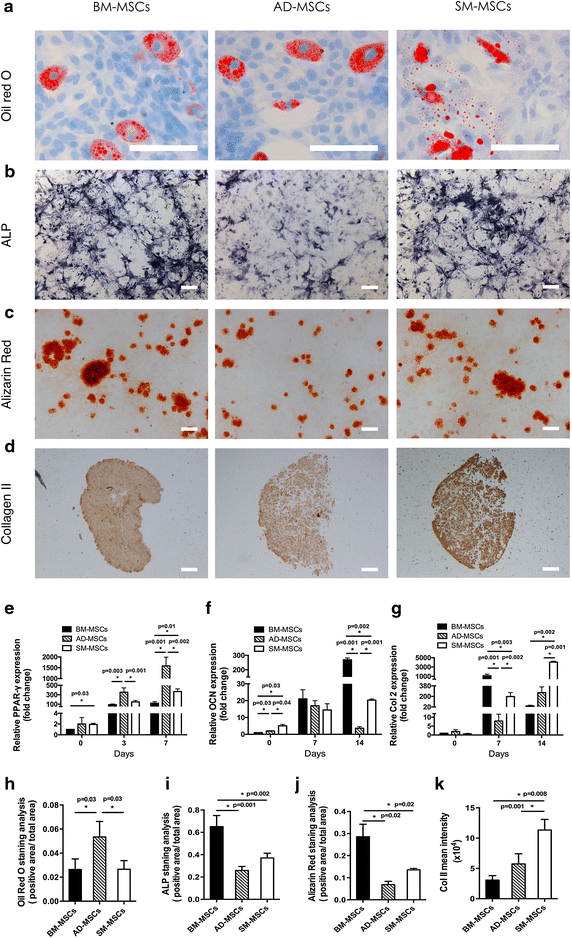
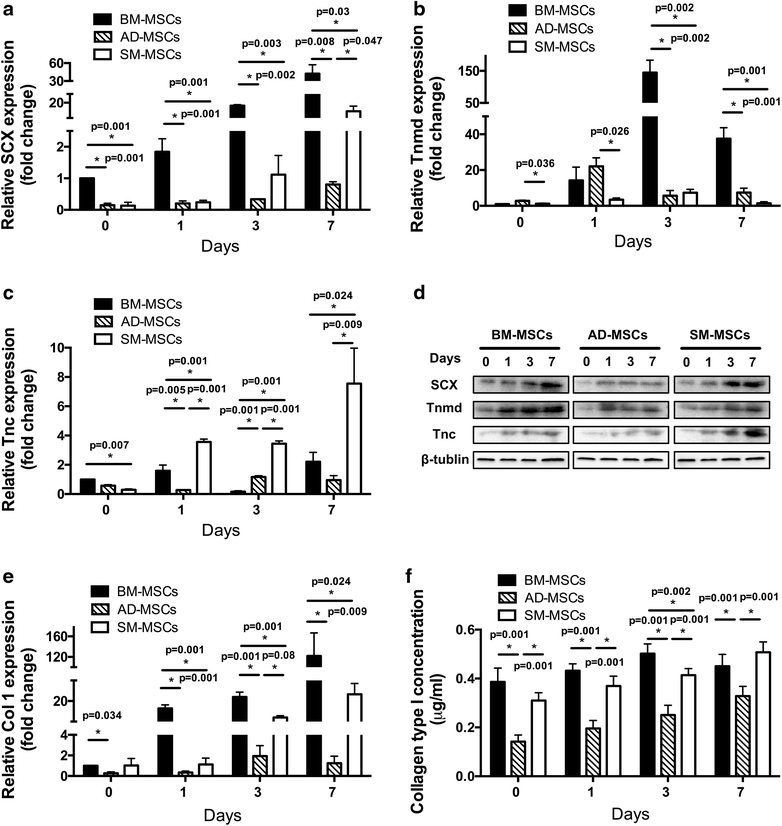
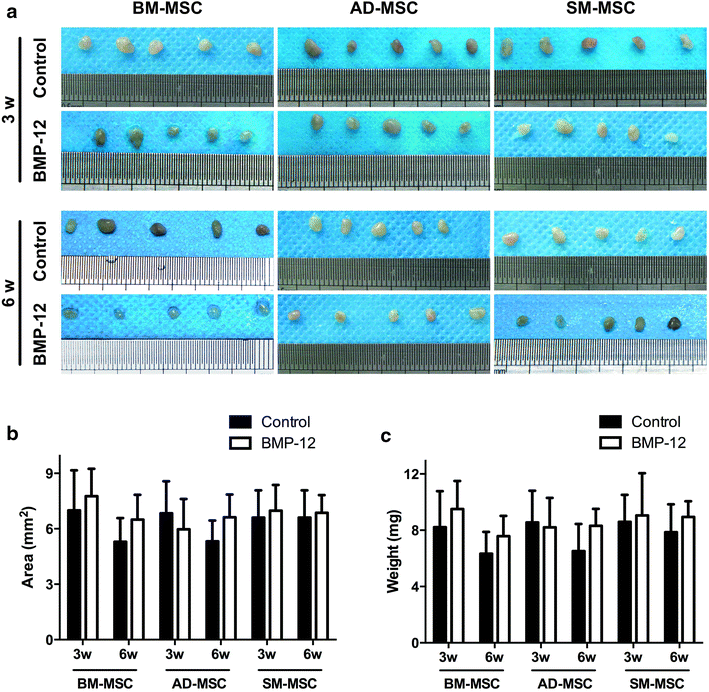
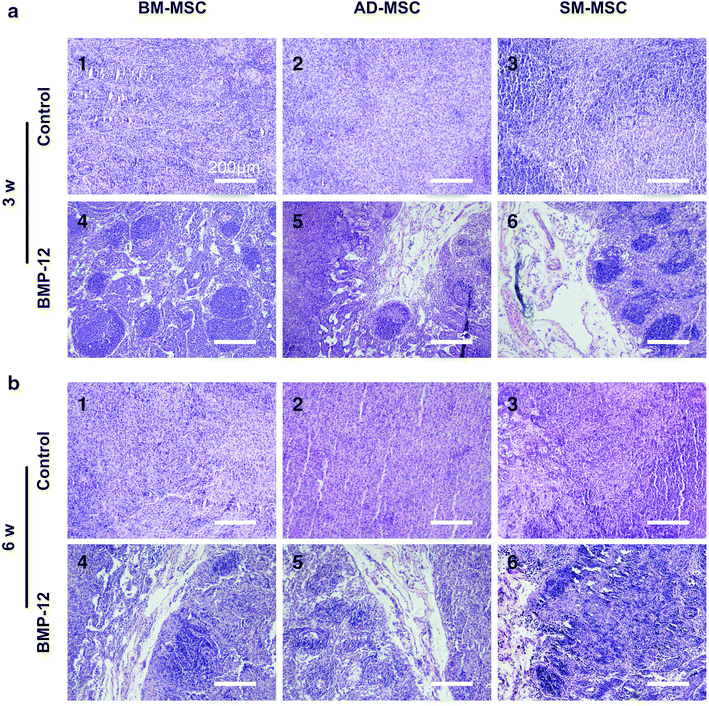
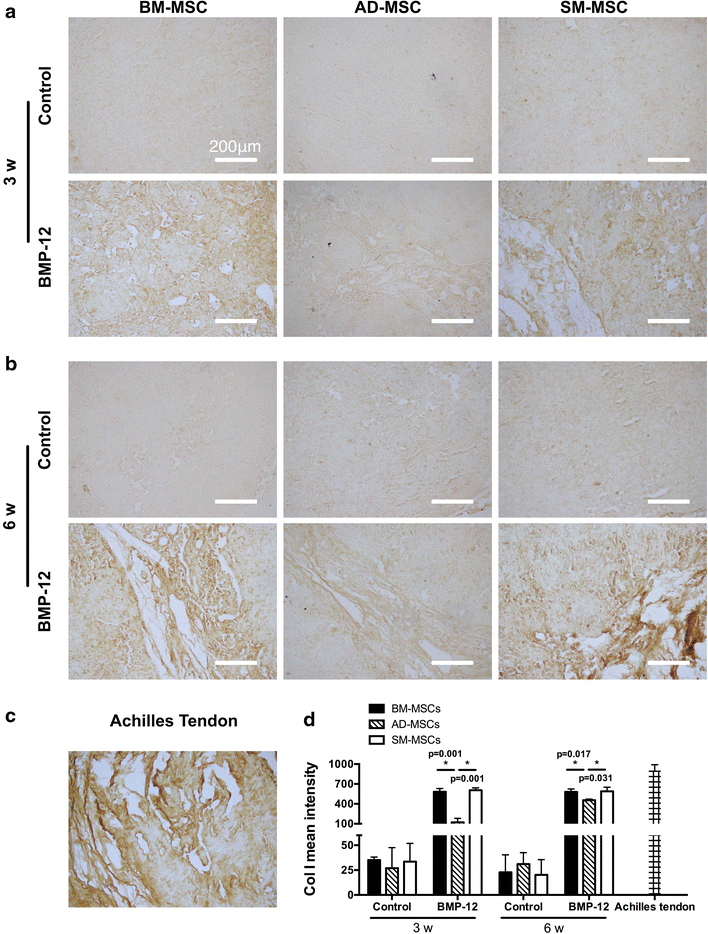
Results: The three types of MSCs exhibited similar fibroblast-like morphology and surface markers but different differentiation potentials toward adipogenic, osteogenic, and chondrogenic lineage fates. Bone marrow-derived MSCs (BM-MSCs) showed the most superior in vitro tenogenic differentiation capacity, followed by synovial membrane-derived MSCs (SM-MSCs) and then adipose-derived MSCs (AD-MSCs). After implantation, all three types of MSC masses infected with BMP-12 recombinant adenovirus emerged in the form of fiber-like matrix, especially in 6-week specimens, compared with the control MSCs in vivo. BM-MSCs and SM-MSCs revealed more intense staining for collagen type I (Col I) compared with AD-MSCs. Differences were not observed between BM-MSCs and SM-MSCs. However, SM-MSCs demonstrated higher proliferation capacity than BM-MSCs.
Conclusion: BM-MSCs exhibited the most superior tenogenic differentiation capacity, followed by SM-MSCs. By contrast, AD-MSCs demonstrated the inferior capacity among the three types of MSCs in the presence of BMP-12 both in vivo and in vitro.
Figures

References
-
- Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. - PubMed
-
- Hankemeier S, Keus M, Zeichen J, Jagodzinski M, Barkhausen T, Bosch U, et al. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005;11:41–49. doi: 10.1089/ten.2005.11.41. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

