Related Mechanisms of Antibody Somatic Hypermutation and Class Switch Recombination
- PMID: 26104555
- PMCID: PMC4481323
- DOI: 10.1128/microbiolspec.MDNA3-0037-2014
Related Mechanisms of Antibody Somatic Hypermutation and Class Switch Recombination
Abstract
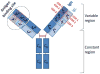
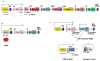
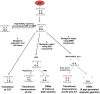
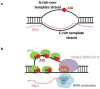
The primary antibody repertoire is generated by mechanisms involving the assembly of the exons that encode the antigen-binding variable regions of immunoglobulin heavy (IgH) and light (IgL) chains during the early development of B lymphocytes. After antigen-dependent activation, mature B lymphocytes can further alter their IgH and IgL variable region exons by the process of somatic hypermutation (SHM), which allows the selection of B cells in which SHMs resulted in the production of antibodies with increased antigen affinity. In addition, during antigen-dependent activation, B cells can also change the constant region of their IgH chain through a DNA double-strand-break (DSB) dependent process referred to as IgH class switch recombination (CSR), which generates B cell progeny that produce antibodies with different IgH constant region effector functions that are best suited for a elimination of a particular pathogen or in a particular setting. Both the mutations that underlie SHM and the DSBs that underlie CSR are initiated in target genes by activation-induced cytidine deaminase (AID). This review describes in depth the processes of SHM and CSR with a focus on mechanisms that direct AID cytidine deamination in activated B cells and mechanisms that promote the differential outcomes of such cytidine deamination.
Figures


References
-
- Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28(5):609–619. - PubMed
-
- Cobb RM, Oestreich KJ, Osipovich OA, Oltz EM. Accessibility control of V(D)J recombination. Adv Immunol. 2006;91:45–109. - PubMed
-
- Pan-Hammarstrom Q, Zhao Y, Hammarstrom L. Class switch recombination: a comparison between mouse and human. Adv Immunol. 93:1–61. - PubMed
-
- Schatz DG, Swanson PC. V(D)J recombination: mechanisms of initiation. Annu Rev Genet. 2011;45:167–202. - PubMed
-
- Deriano L, Roth DB. Modernizing the nonhomologous end-joining repertoire: alternative and classical NHEJ share the stage. Annu Rev Genet. 2013;47:433–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

