Topological Behavior of Plasmid DNA
- PMID: 26104708
- PMCID: PMC4480603
- DOI: 10.1128/microbiolspec.PLAS-0036-2014
Topological Behavior of Plasmid DNA
Abstract
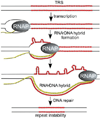
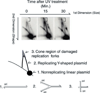
The discovery of the B-form structure of DNA by Watson and Crick led to an explosion of research on nucleic acids in the fields of biochemistry, biophysics, and genetics. Powerful techniques were developed to reveal a myriad of different structural conformations that change B-DNA as it is transcribed, replicated, and recombined and as sister chromosomes are moved into new daughter cell compartments during cell division. This article links the original discoveries of superhelical structure and molecular topology to non-B form DNA structure and contemporary biochemical and biophysical techniques. The emphasis is on the power of plasmids for studying DNA structure and function. The conditions that trigger the formation of alternative DNA structures such as left-handed Z-DNA, inter- and intra-molecular triplexes, triple-stranded DNA, and linked catenanes and hemicatenanes are explained. The DNA dynamics and topological issues are detailed for stalled replication forks and for torsional and structural changes on DNA in front of and behind a transcription complex and a replisome. The complex and interconnected roles of topoisomerases and abundant small nucleoid association proteins are explained. And methods are described for comparing in vivo and in vitro reactions to probe and understand the temporal pathways of DNA and chromosome chemistry that occur inside living cells.
Conflict of interest statement
Conflicts of interest: We declare no conflicts.
Figures













References
-
- Bauer WR, Crick FHC, White JH. Supercoiled DNA. Sci Am. 1980;243:100–113. - PubMed
-
- Calugareanu G. Sur las classes d’isotopie des noeuds tridimensionnels et leurs invariants. Czech Math J. 1961;11:588–625.
-
- White JH. Self-linking and the Gauss integral in higher dimensions. Am J Math. 1969;91:693–728.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

