Serine Resolvases
- PMID: 26104713
- PMCID: PMC5659196
- DOI: 10.1128/microbiolspec.MDNA3-0045-2014
Serine Resolvases
Abstract
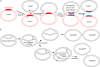
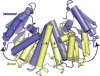
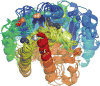
Serine resolvases are an interesting group of site-specific recombinases that, in their native contexts, resolve large fused replicons into smaller separated ones. Some resolvases are encoded by replicative transposons and resolve the transposition product, in which the donor and recipient molecules are fused, into separate replicons. Other resolvases are encoded by plasmids and function to resolve plasmid dimers into monomers. Both types are therefore involved in the spread and maintenance of antibiotic-resistance genes. Resolvases and the closely related invertases were the first serine recombinases to be studied in detail, and much of our understanding of the unusual strand exchange mechanism of serine recombinases is owed to those early studies. Resolvases and invertases have also served as paradigms for understanding how DNA topology can be harnessed to regulate enzyme activity. Finally, their relatively modular structure, combined with a wealth of structural and biochemical data, has made them good choices for engineering chimeric recombinases with designer specificity. This chapter focuses on the current understanding of serine resolvases, with a focus on the contributions of structural studies.
Figures
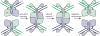

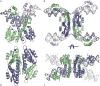
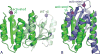

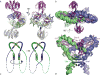
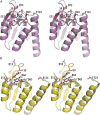
References
-
- Grindley NDF, Whiteson KL, Rice PA. Mechanisms of site-specific recombination. Annu Rev Biochem. 2006;75:567–605. - PubMed
-
- Boocock MR, Brown JL, Sherratt DJ. Structural and catalytic properties of specific complexes between Tn3 resolvase and the recombination site res. Biochem Soc Trans. 1986;14:214–216. - PubMed
-
- Wasserman SA, Dungan JM, Cozzarelli NR. Discovery of a predicted DNA knot substantiates a model for site-specific recombination. Science. 1985;229:171–174. - PubMed
-
- Merickel SK, Johnson RC. Topological analysis of Hin-catalysed DNA recombination in vivo and in vitro. Mol Microbiol. 2004;51:1143–1154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

