Mechanisms of DNA Transposition
- PMID: 26104718
- PMCID: PMC7422641
- DOI: 10.1128/microbiolspec.MDNA3-0034-2014
Mechanisms of DNA Transposition
Abstract
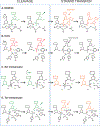
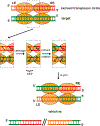
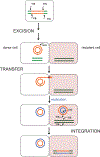
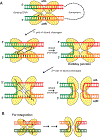
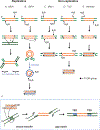
DNA transposases use a limited repertoire of structurally and mechanistically distinct nuclease domains to catalyze the DNA strand breaking and rejoining reactions that comprise DNA transposition. Here, we review the mechanisms of the four known types of transposition reactions catalyzed by (1) RNase H-like transposases (also known as DD(E/D) enzymes); (2) HUH single-stranded DNA transposases; (3) serine transposases; and (4) tyrosine transposases. The large body of accumulated biochemical and structural data, particularly for the RNase H-like transposases, has revealed not only the distinguishing features of each transposon family, but also some emerging themes that appear conserved across all families. The more-recently characterized single-stranded DNA transposases provide insight into how an ancient HUH domain fold has been adapted for transposition to accomplish excision and then site-specific integration. The serine and tyrosine transposases are structurally and mechanistically related to their cousins, the serine and tyrosine site-specific recombinases, but have to date been less intensively studied. These types of enzymes are particularly intriguing as in the context of site-specific recombination they require strict homology between recombining sites, yet for transposition can catalyze the joining of transposon ends to form an excised circle and then integration into a genomic site with much relaxed sequence specificity.
Figures
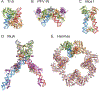

References
-
- Curcio MJ, Derbyshire KM. 2003. The outs and ins of transposition: From Mu to kangaroo. Nature Rev Mol Cell Biol 4:865–877. - PubMed
-
- Dyda F, Hickman AB, Jenkins TM, Engelman A, Craigie R,Davies DR. 1994. Crystal structure of the catalytic domain of HIV-1 integrase: Similarity to other polynucleotidyl transferases. Science 266: 1981–1986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

