How a patient advocacy group developed the first proposed draft guidance document for industry for submission to the U.S. Food and Drug Administration
- PMID: 26104810
- PMCID: PMC4486430
- DOI: 10.1186/s13023-015-0281-2
How a patient advocacy group developed the first proposed draft guidance document for industry for submission to the U.S. Food and Drug Administration
Abstract
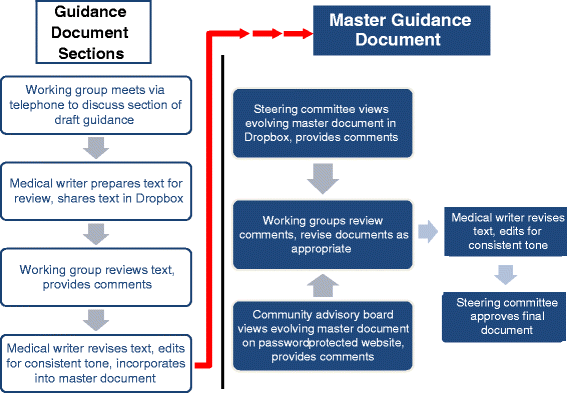
Among the challenges confronting patients with rare diseases is a dearth of treatment options. The development of safe and effective new therapies is hampered by challenges associated with conducting clinical trials in small populations. In this article, we describe how the Duchenne muscular dystrophy community-led by Parent Project Muscular Dystrophy-created a proposed draft guidance document for industry for submission to the U.S. Food and Drug Administration. This unprecedented undertaking involved a broad coalition of more than 80 stakeholders collaborating across nine time zones to produce a document in only 6 months. We hope that other rare disease communities and advocacy organizations can use our experience as a model for developing their own draft guidance documents.
Figures
Similar articles
-
A community-engaged approach to quantifying caregiver preferences for the benefits and risks of emerging therapies for Duchenne muscular dystrophy.Clin Ther. 2014 May;36(5):624-37. doi: 10.1016/j.clinthera.2014.04.011. Clin Ther. 2014. PMID: 24852596
-
Towards regulatory endorsement of drug development tools to promote the application of model-informed drug development in Duchenne muscular dystrophy.J Pharmacokinet Pharmacodyn. 2019 Oct;46(5):441-455. doi: 10.1007/s10928-019-09642-7. Epub 2019 May 24. J Pharmacokinet Pharmacodyn. 2019. PMID: 31127458
-
Duchenne muscular dystrophy: Drug development and regulatory considerations.Muscle Nerve. 2010 Jun;41(6):740-5. doi: 10.1002/mus.21623. Muscle Nerve. 2010. PMID: 20373504
-
Stakeholder cooperation to overcome challenges in orphan medicine development: the example of Duchenne muscular dystrophy.Lancet Neurol. 2016 Jul;15(8):882-890. doi: 10.1016/S1474-4422(16)30035-7. Lancet Neurol. 2016. PMID: 27302365 Review.
-
The evolution of patient-focused drug development and Duchenne muscular dystrophy.Expert Rev Pharmacoecon Outcomes Res. 2020 Feb;20(1):57-68. doi: 10.1080/14737167.2020.1734454. Epub 2020 Mar 6. Expert Rev Pharmacoecon Outcomes Res. 2020. PMID: 32098551 Review.
Cited by
-
Stimulating Patient Engagement in Medical Device Development in Kidney Disease: A Report of a Kidney Health Initiative Workshop.Am J Kidney Dis. 2017 Oct;70(4):561-569. doi: 10.1053/j.ajkd.2017.03.013. Epub 2017 Apr 27. Am J Kidney Dis. 2017. PMID: 28457656 Free PMC article.
-
A Framework for Instrument Development of a Choice Experiment: An Application to Type 2 Diabetes.Patient. 2016 Oct;9(5):465-79. doi: 10.1007/s40271-016-0170-3. Patient. 2016. PMID: 27120338
-
A Comparison of Caregiver and Patient Preferences for Treating Duchenne Muscular Dystrophy.Patient. 2022 Sep;15(5):577-588. doi: 10.1007/s40271-022-00574-y. Epub 2022 Mar 4. Patient. 2022. PMID: 35243571 Free PMC article.
-
Can Innovative Trial Designs in Orphan Diseases Drive Advancement of Treatments for Common Neurological Diseases?Clin Pharmacol Ther. 2022 Apr;111(4):799-806. doi: 10.1002/cpt.2528. Epub 2022 Feb 7. Clin Pharmacol Ther. 2022. PMID: 35034352 Free PMC article. Review.
-
Life with a Primary Immune Deficiency: a Systematic Synthesis of the Literature and Proposed Research Agenda.J Clin Immunol. 2016 Feb;36(2):123-33. doi: 10.1007/s10875-016-0241-1. Epub 2016 Feb 12. J Clin Immunol. 2016. PMID: 26873708 Free PMC article.
References
-
- Muscular Dystrophy Association. Duchenne muscular dystrophy: overview [Internet]. Chicago, IL: MDA Inc; http://mda.org/disease/duchenne-muscular-dystrophy/overview Accessed 16 Dec 2014.
-
- National Institute of Neurological Disorders and Stroke . Muscular dystrophy: hope through research [Internet] Bethesda, MD: NINDS; 2014.
-
- Centers for Disease Control and Prevention Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years–four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;40:1119–22. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources