Preoperative Single-Fraction Partial Breast Radiation Therapy: A Novel Phase 1, Dose-Escalation Protocol With Radiation Response Biomarkers
- PMID: 26104938
- PMCID: PMC4481883
- DOI: 10.1016/j.ijrobp.2015.03.007
Preoperative Single-Fraction Partial Breast Radiation Therapy: A Novel Phase 1, Dose-Escalation Protocol With Radiation Response Biomarkers
Abstract
Purpose: Women with biologically favorable early-stage breast cancer are increasingly treated with accelerated partial breast radiation (PBI). However, treatment-related morbidities have been linked to the large postoperative treatment volumes required for external beam PBI. Relative to external beam delivery, alternative PBI techniques require equipment that is not universally available. To address these issues, we designed a phase 1 trial utilizing widely available technology to 1) evaluate the safety of a single radiation treatment delivered preoperatively to the small-volume, intact breast tumor and 2) identify imaging and genomic markers of radiation response.
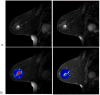
Methods and materials: Women aged ≥55 years with clinically node-negative, estrogen receptor-positive, and/or progesterone receptor-positive HER2-, T1 invasive carcinomas, or low- to intermediate-grade in situ disease ≤2 cm were enrolled (n=32). Intensity modulated radiation therapy was used to deliver 15 Gy (n=8), 18 Gy (n=8), or 21 Gy (n=16) to the tumor with a 1.5-cm margin. Lumpectomy was performed within 10 days. Paired pre- and postradiation magnetic resonance images and patient tumor samples were analyzed.
Results: No dose-limiting toxicity was observed. At a median follow-up of 23 months, there have been no recurrences. Physician-rated cosmetic outcomes were good/excellent, and chronic toxicities were grade 1 to 2 (fibrosis, hyperpigmentation) in patients receiving preoperative radiation only. Evidence of dose-dependent changes in vascular permeability, cell density, and expression of genes regulating immunity and cell death were seen in response to radiation.
Conclusions: Preoperative single-dose radiation therapy to intact breast tumors is well tolerated. Radiation response is marked by early indicators of cell death in this biologically favorable patient cohort. This study represents a first step toward a novel partial breast radiation approach. Preoperative radiation should be tested in future clinical trials because it has the potential to challenge the current treatment paradigm and provide a path forward to identify radiation response biomarkers.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) International journal of radiation oncology, biology, physics. 2009 Jul 15;74(4):987–1001. - PubMed
-
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014 Feb 15;383(9917):603–613. - PubMed
-
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. The lancet oncology. 2013 Dec;14(13):1269–1277. - PubMed
-
- Julian TB, Costantino JP, Vicini FA, et al. Early toxicity results with 3D conformal external beam therapy (CEBT) from the NSABP B-39/RTOG 0413 accelerated partial breast irradiation (APBI) trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(suppl; abstr 1011) suppl; abstr 1011.
-
- Hepel JT, Tokita M, MacAusland SG, et al. Toxicity of three-dimensional conformal radiotherapy for accelerated partial breast irradiation. International journal of radiation oncology, biology, physics. 2009 Dec 1;75(5):1290–1296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

