Consensus Paper: Revisiting the Symptoms and Signs of Cerebellar Syndrome
- PMID: 26105056
- PMCID: PMC5565264
- DOI: 10.1007/s12311-015-0687-3
Consensus Paper: Revisiting the Symptoms and Signs of Cerebellar Syndrome
Abstract
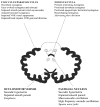
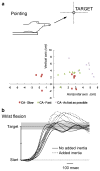
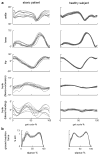
The cerebellum is involved in sensorimotor operations, cognitive tasks and affective processes. Here, we revisit the concept of the cerebellar syndrome in the light of recent advances in our understanding of cerebellar operations. The key symptoms and signs of cerebellar dysfunction, often grouped under the generic term of ataxia, are discussed. Vertigo, dizziness, and imbalance are associated with lesions of the vestibulo-cerebellar, vestibulo-spinal, or cerebellar ocular motor systems. The cerebellum plays a major role in the online to long-term control of eye movements (control of calibration, reduction of eye instability, maintenance of ocular alignment). Ocular instability, nystagmus, saccadic intrusions, impaired smooth pursuit, impaired vestibulo-ocular reflex (VOR), and ocular misalignment are at the core of oculomotor cerebellar deficits. As a motor speech disorder, ataxic dysarthria is highly suggestive of cerebellar pathology. Regarding motor control of limbs, hypotonia, a- or dysdiadochokinesia, dysmetria, grasping deficits and various tremor phenomenologies are observed in cerebellar disorders to varying degrees. There is clear evidence that the cerebellum participates in force perception and proprioceptive sense during active movements. Gait is staggering with a wide base, and tandem gait is very often impaired in cerebellar disorders. In terms of cognitive and affective operations, impairments are found in executive functions, visual-spatial processing, linguistic function, and affective regulation (Schmahmann's syndrome). Nonmotor linguistic deficits including disruption of articulatory and graphomotor planning, language dynamics, verbal fluency, phonological, and semantic word retrieval, expressive and receptive syntax, and various aspects of reading and writing may be impaired after cerebellar damage. The cerebellum is organized into (a) a primary sensorimotor region in the anterior lobe and adjacent part of lobule VI, (b) a second sensorimotor region in lobule VIII, and (c) cognitive and limbic regions located in the posterior lobe (lobule VI, lobule VIIA which includes crus I and crus II, and lobule VIIB). The limbic cerebellum is mainly represented in the posterior vermis. The cortico-ponto-cerebellar and cerebello-thalamo-cortical loops establish close functional connections between the cerebellum and the supratentorial motor, paralimbic and association cortices, and cerebellar symptoms are associated with a disruption of these loops.
Keywords: A- or Dysdiadochokinesia; Affect; Ataxia; Cerebellar syndrome; Cerebellum; Cognition; Dysarthria; Dysmetria; Eye movements; Functional topography; Hypotonia; Language; Loops; Speech; Tremor.
Conflict of interest statement
Figures


References
-
- Grimaldi G, Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11(2):336–51. - PubMed
-
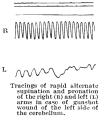
- Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain. 1917;40:461–535.
-
- Babinski J. Sur le role du cervelet dans les actes volitionnels nécessitant une succession rapide de mouvements (1) (diadococinésie) Rev Neurol. 1902;10:1013–5.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

