Structural analysis of a class III preQ1 riboswitch reveals an aptamer distant from a ribosome-binding site regulated by fast dynamics
- PMID: 26106162
- PMCID: PMC4500280
- DOI: 10.1073/pnas.1503955112
Structural analysis of a class III preQ1 riboswitch reveals an aptamer distant from a ribosome-binding site regulated by fast dynamics
Abstract
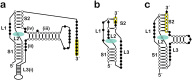
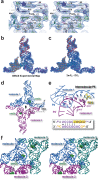
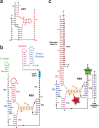
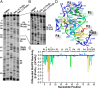
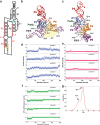
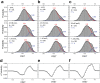
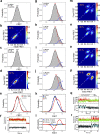
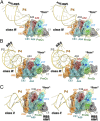
PreQ1-III riboswitches are newly identified RNA elements that control bacterial genes in response to preQ1 (7-aminomethyl-7-deazaguanine), a precursor to the essential hypermodified tRNA base queuosine. Although numerous riboswitches fold as H-type or HLout-type pseudoknots that integrate ligand-binding and regulatory sequences within a single folded domain, the preQ1-III riboswitch aptamer forms a HLout-type pseudoknot that does not appear to incorporate its ribosome-binding site (RBS). To understand how this unusual organization confers function, we determined the crystal structure of the class III preQ1 riboswitch from Faecalibacterium prausnitzii at 2.75 Å resolution. PreQ1 binds tightly (KD,app 6.5 ± 0.5 nM) between helices P1 and P2 of a three-way helical junction wherein the third helix, P4, projects orthogonally from the ligand-binding pocket, exposing its stem-loop to base pair with the 3' RBS. Biochemical analysis, computational modeling, and single-molecule FRET imaging demonstrated that preQ1 enhances P4 reorientation toward P1-P2, promoting a partially nested, H-type pseudoknot in which the RBS undergoes rapid docking (kdock ∼ 0.6 s(-1)) and undocking (kundock ∼ 1.1 s(-1)). Discovery of such dynamic conformational switching provides insight into how a riboswitch with bipartite architecture uses dynamics to modulate expression platform accessibility, thus expanding the known repertoire of gene control strategies used by regulatory RNAs.
Keywords: crystal structure; gene regulation; molecular dynamics; preQ1 riboswitch; single-molecule FRET.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



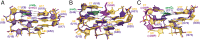
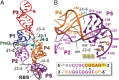



References
-
- Sudarsan N, Cohen-Chalamish S, Nakamura S, Emilsson GM, Breaker RR. Thiamine pyrophosphate riboswitches are targets for the antimicrobial compound pyrithiamine. Chem Biol. 2005;12(12):1325–1335. - PubMed
-
- Blount KF, Wang JX, Lim J, Sudarsan N, Breaker RR. Antibacterial lysine analogs that target lysine riboswitches. Nat Chem Biol. 2007;3(1):44–49. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

