Transient receptor potential cation channel subfamily V member 1 expressing corneal sensory neurons can be subdivided into at least three subpopulations
- PMID: 26106303
- PMCID: PMC4458692
- DOI: 10.3389/fnana.2015.00071
Transient receptor potential cation channel subfamily V member 1 expressing corneal sensory neurons can be subdivided into at least three subpopulations
Abstract
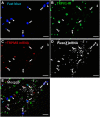
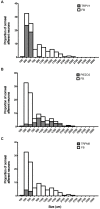
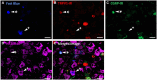
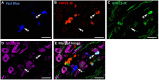
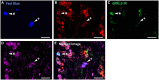
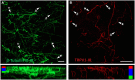
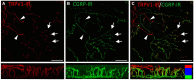
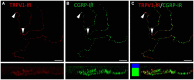
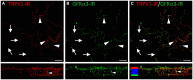
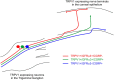
The cornea is innervated by three main functional classes of sensory neurons: polymodal nociceptors, pure mechano-nociceptors and cold-sensing neurons. Here we explored transient receptor potential cation channel subfamily V member 1 (TRPV1) expression in guinea pig corneal sensory neurons, a widely used molecular marker of polymodal nociceptors. We used retrograde tracing to identify corneal afferent neurons in the trigeminal ganglion (TG) and double label in situ hybridization and/or immunohistochemistry to determine their molecular profile. In addition, we used immunohistochemistry to reveal the neurochemistry and structure of TRPV1 expressing nerve endings in the corneal epithelium. Approximately 45% of corneal afferent neurons expressed TRPV1, 28% expressed Piezo2 (a marker of putative pure mechano-nociceptors) and 8% expressed the transient receptor potential cation channel subfamily M member 8 (TRPM8; a marker of cold-sensing neurons). There was no co-expression of TRPV1 and Piezo2 in corneal afferent neurons, but 6% of TRPV1 neurons co-expressed TRPM8. The TRPV1 expressing corneal afferent neurons could be divided into three subpopulations on the basis of calcitonin gene-related peptide (CGRP) and/or or glial cell line-derived neurotrophic factor family receptor alpha3 (GFRα3) co-expression. In the corneal epithelium, the TRPV1 axons that co-expressed CGRP and GFRα3 ended as simple unbranched endings in the wing cell layer. In contrast, those that only co-expressed GFRα3 had ramifying endings that branched and terminated in the squamous cell layer, whereas those that only co-expressed CGRP had simple endings in the basal epithelium. This study shows that the majority of TRPV1 expressing corneal afferent neurons (>90%) are likely to be polymodal nociceptors. Furthermore, TRPV1 expressing corneal afferent neurons can be subdivided into specific subpopulations based on their molecular phenotype, nerve terminal morphology and distribution in the corneal epithelium.
Keywords: TRPV1; cornea; polymodal nociceptor; primary afferent neurons; sensory neurons.
Figures
References
-
- Belmonte C., Gallar J. (1996). “Corneal nociceptors,” in Neurobiology of Nociceptors, eds Belmonte C., Cervero F. (New York: Oxford University Press; ), 147–183.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

