Facing Challenges in Differential Classical Conditioning Research: Benefits of a Hybrid Design for Simultaneous Electrodermal and Electroencephalographic Recording
- PMID: 26106318
- PMCID: PMC4460875
- DOI: 10.3389/fnhum.2015.00336
Facing Challenges in Differential Classical Conditioning Research: Benefits of a Hybrid Design for Simultaneous Electrodermal and Electroencephalographic Recording
Abstract
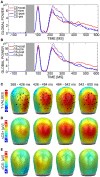
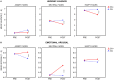
Several challenges make it difficult to simultaneously investigate central and autonomous nervous system correlates of conditioned stimulus (CS) processing in classical conditioning paradigms. Such challenges include, for example, the discrepant requirements of electroencephalography (EEG) and electrodermal activity (EDA) recordings with regard to multiple repetitions of conditions and sufficient trial duration. Here, we propose a MultiCS conditioning set-up, in which we increased the number of CSs, decreased the number of learning trials, and used trials of short and long durations for meeting requirements of simultaneous EEG-EDA recording in a differential aversive conditioning task. Forty-eight participants underwent MultiCS conditioning, in which four neutral faces (CS+) were paired four times each with aversive electric stimulation (unconditioned stimulus) during acquisition, while four different neutral faces (CS-) remained unpaired. When comparing after relative to before learning measurements, EEG revealed an enhanced centro-posterior positivity to CS+ vs. CS- during 368-600 ms, and subjective ratings indicated CS+ to be less pleasant and more arousing than CS-. Furthermore, changes in CS valence and arousal were strong enough to bias subjective ratings when faces of CS+/CS- identity were displayed with different emotional expression (happy, angry) in a post-experimental behavioral task. In contrast to a persistent neural and evaluative CS+/CS- differentiation that sustained multiple unreinforced CS presentations, electrodermal differentiation was rapidly extinguished. Current results suggest that MultiCS conditioning provides a promising paradigm for investigating pre-post-learning changes under minimal influences of extinction and overlearning of simple stimulus features. Our data also revealed methodological pitfalls, such as the possibility of occurring artifacts when combining different acquisition systems for central and peripheral psychophysiological measures.
Keywords: EEG; MultiCS conditioning; affective learning; emotion; skin conductance.
Figures


Similar articles
-
Identity and expression processing during classical conditioning with faces.Psychophysiology. 2018 Oct;55(10):e13203. doi: 10.1111/psyp.13203. Epub 2018 Aug 2. Psychophysiology. 2018. PMID: 30069886
-
Conditional stimulus choices affect fear learning: Comparing fear conditioning with neutral faces and shapes or angry faces.Psychophysiology. 2022 Oct;59(10):e14068. doi: 10.1111/psyp.14068. Epub 2022 Apr 27. Psychophysiology. 2022. PMID: 35477888 Free PMC article.
-
Classical conditioning in borderline personality disorder: an fMRI study.Eur Arch Psychiatry Clin Neurosci. 2016 Jun;266(4):291-305. doi: 10.1007/s00406-015-0593-1. Epub 2015 Mar 27. Eur Arch Psychiatry Clin Neurosci. 2016. PMID: 25814470
-
The need for standards in the design of differential fear conditioning and extinction experiments in youth: A systematic review and recommendations for research on anxiety.Behav Res Ther. 2019 Jan;112:42-62. doi: 10.1016/j.brat.2018.11.009. Epub 2018 Nov 20. Behav Res Ther. 2019. PMID: 30502721
-
Emotion Regulation Assessment: A New Perspective Using Simultaneous Electroencephalographic and Electrodermal Recordings.Clin EEG Neurosci. 2025 Jul;56(4):295-304. doi: 10.1177/15500594241302553. Epub 2024 Dec 10. Clin EEG Neurosci. 2025. PMID: 39655572 Review.
Cited by
-
Transcutaneous Auricular Vagus Nerve Stimulation Enhances Emotional Processing and Long-Term Recognition Memory: Electrophysiological Evidence Across Two Studies.Psychophysiology. 2025 Mar;62(3):e70034. doi: 10.1111/psyp.70034. Psychophysiology. 2025. PMID: 40066789 Free PMC article.
-
Neurophysiological Effects Associated With Subliminal Conditioning of Appetite Motivations.Front Psychol. 2019 Mar 5;10:457. doi: 10.3389/fpsyg.2019.00457. eCollection 2019. Front Psychol. 2019. PMID: 30890986 Free PMC article.
-
Establishment of Emotional Memories Is Mediated by Vagal Nerve Activation: Evidence from Noninvasive taVNS.J Neurosci. 2021 Sep 8;41(36):7636-7648. doi: 10.1523/JNEUROSCI.2329-20.2021. Epub 2021 Jul 19. J Neurosci. 2021. PMID: 34281991 Free PMC article.
-
Anticipatory representations of reward and threat in perceptual areas from preadolescence to late adolescence.Dev Cogn Neurosci. 2017 Jun;25:246-259. doi: 10.1016/j.dcn.2017.03.001. Epub 2017 Mar 12. Dev Cogn Neurosci. 2017. PMID: 28359682 Free PMC article.
-
The excessive generalization of fear affected by perceptual bias in experimental pain individuals: Evidence from an event-related potential study.Brain Behav. 2023 Jul;13(7):e3050. doi: 10.1002/brb3.3050. Epub 2023 May 3. Brain Behav. 2023. PMID: 37132353 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

