A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone)
- PMID: 26106644
- PMCID: PMC4461790
- DOI: 10.1155/2015/816364
A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone)
Abstract
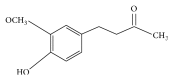
Humans have been using natural products for medicinal use for ages. Natural products of therapeutic importance are compounds derived from plants, animals, or any microorganism. Ginger is also one of the most commonly used condiments and a natural drug in vogue. It is a traditional medicine, having some active ingredients used for the treatment of numerous diseases. During recent research on ginger, various ingredients like zingerone, shogaol, and paradol have been obtained from it. Zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone) is a nontoxic and inexpensive compound with varied pharmacological activities. It is the least pungent component of Zingiber officinale. Zingerone is absent in fresh ginger but cooking or heating transforms gingerol to zingerone. Zingerone closely related to vanillin from vanilla and eugenol from clove. Zingerone has potent anti-inflammatory, antidiabetic, antilipolytic, antidiarrhoeic, antispasmodic, and so forth properties. Besides, it displays the property of enhancing growth and immune stimulation. It behaves as appetite stimulant, anxiolytic, antithrombotic, radiation protective, and antimicrobial. Also, it inhibits the reactive nitrogen species which are important in causing Alzheimer's disease and many other disorders. This review is written to shed light on the various pharmacological properties of zingerone and its role in alleviating numerous human and animal diseases.
Figures
References
-
- Zhang Y.-X., Li J.-S., Chen L.-H., Peng W.-W., Cai B.-C. Simultaneous determination of five gingerols in raw and processed ginger by HPLC. Chinese Pharmaceutical Journal. 2012;47(6):471–474.
-
- Cotton W. J. Production of zingerone. Google Patents, 1945.
-
- Takizawa M., Sato M., Kusuoku H., Sakasai M. Lipolysis stimulator. Google Patents, 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical