Favorable alteration of tumor microenvironment by immunomodulatory cytokines for efficient T-cell therapy in solid tumors
- PMID: 26107883
- PMCID: PMC4479879
- DOI: 10.1371/journal.pone.0131242
Favorable alteration of tumor microenvironment by immunomodulatory cytokines for efficient T-cell therapy in solid tumors
Abstract
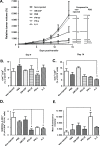
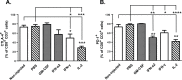
Unfavorable ratios between the number and activation status of effector and suppressor immune cells infiltrating the tumor contribute to resistance of solid tumors to T-cell based therapies. Here, we studied the capacity of FDA and EMA approved recombinant cytokines to manipulate this balance in favor of efficient anti-tumor responses in B16.OVA melanoma bearing C57BL/6 mice. Intratumoral administration of IFN-α2, IFN-γ, TNF-α, and IL-2 significantly enhanced the anti-tumor effect of ovalbumin-specific CD8+ T-cell (OT-I) therapy, whereas GM-CSF increased tumor growth in association with an increase in immunosuppressive cell populations. None of the cytokines augmented tumor trafficking of OT-I cells significantly, but injections of IFN-α2, IFN-γ and IL-2 increased intratumoral cytokine secretion and recruitment of endogenous immune cells capable of stimulating T-cells, such as natural killer and maturated CD11c+ antigen-presenting cells. Moreover, IFN-α2 and IL-2 increased the levels of activated tumor-infiltrating CD8+ T-cells concomitant with reduction in the CD8+ T-cell expression of anergy markers CTLA-4 and PD-1. In conclusion, intratumoral administration of IFN-α2, IFN-γ and IL-2 can lead to immune sensitization of the established tumor, whereas GM-CSF may contribute to tumor-associated immunosuppression. The results described here provide rationale for including local administration of immunostimulatory cytokines into T-cell therapy regimens. One appealing embodiment of this would be vectored delivery which could be advantageous over direct injection of recombinant molecules with regard to efficacy, cost, persistence and convenience.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

