Using ClinicalTrials.gov to supplement information in ophthalmology conference abstracts about trial outcomes: a comparison study
- PMID: 26107924
- PMCID: PMC4479484
- DOI: 10.1371/journal.pone.0130619
Using ClinicalTrials.gov to supplement information in ophthalmology conference abstracts about trial outcomes: a comparison study
Abstract
Background: Including results from unpublished randomized controlled trials (RCTs) in a systematic review may ameliorate the effect of publication bias in systematic review results. Unpublished RCTs are sometimes described in abstracts presented at conferences, included in trials registers, or both. Trial results may not be available in a trials register and abstracts describing RCT results often lack study design information. Complementary information from a trials register record may be sufficient to allow reliable inclusion of an unpublished RCT only available as an abstract in a systematic review.
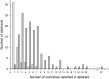
Methods: We identified 496 abstracts describing RCTs presented at the 2007 to 2009 Association for Research in Vision and Ophthalmology (ARVO) meetings; 154 RCTs were registered in ClinicalTrials.gov. Two persons extracted verbatim primary and non-primary outcomes reported in the abstract and ClinicalTrials.gov record. We compared each abstract outcome with all ClinicalTrials.gov outcomes and coded matches as complete, partial, or no match.
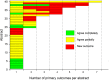
Results: We identified 800 outcomes in 152 abstracts (95 primary [51 abstracts] and 705 [141 abstracts] non-primary outcomes). No outcomes were reported in 2 abstracts. Of 95 primary outcomes, 17 (18%) agreed completely, 53 (56%) partially, and 25 (26%) had no match with a ClinicalTrials.gov primary or non-primary outcome. Among 705 non-primary outcomes, 56 (8%) agreed completely, 205 (29%) agreed partially, and 444 (63%) had no match with a ClinicalTrials.gov primary or non-primary outcome. Among the 258 outcomes partially agreeing, we found additional information on the time when the outcome was measured more often in ClinicalTrials.gov than in the abstract (141/258 (55%) versus 55/258 (21%)). We found no association between the presence of non-matching "new" outcomes and year of registration, time to registry update, industry sponsorship, or multi-center status.
Conclusion: Conference abstracts may be a valuable source of information about results for outcomes of unpublished RCTs that have been registered in ClinicalTrials.gov. Complementary additional descriptive information may be present for outcomes reported in both sources. However, ARVO abstract authors also present outcomes not reported in ClinicalTrials.gov and these may represent analyses not originally planned.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

