IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)Vγ6(+)γδ T cells
- PMID: 26108163
- PMCID: PMC4521288
- DOI: 10.1038/ncomms8464
IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)Vγ6(+)γδ T cells
Abstract
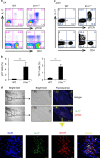
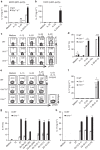
Interleukin-17 (IL-17)-producing γδ T (γδ17) cells have been implicated in inflammatory diseases, but the underlying pathogenic mechanisms remain unclear. Here, we show that both CD4(+) and γδ17 cells are required for the development of autoimmune arthritis in IL-1 receptor antagonist (IL-1Ra)-deficient mice. Specifically, activated CD4(+) T cells direct γδ T-cell infiltration by inducing CCL2 expression in joints. Furthermore, IL-17 reporter mice reveal that the Vγ6(+) subset of CCR2(+) γδ T cells preferentially produces IL-17 in inflamed joints. Importantly, because IL-1Ra normally suppresses IL-1R expression on γδ T cells, IL-1Ra-deficient mice exhibit elevated IL-1R expression on Vγ6(+) cells, which play a critical role in inducing them to produce IL-17. Our findings demonstrate a pathogenic mechanism in which adaptive and innate immunity induce an autoimmune disease in a coordinated manner.
Figures





References
-
- Iwakura Y., Ishigame H., Saijo S. & Nakae S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011). - PubMed
-
- Cua D. J. & Tato C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 (2010). - PubMed
-
- Sutton C. E. et al.. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

