Sampling circulating tumor cells for clinical benefits: how frequent?
- PMID: 26108208
- PMCID: PMC4488127
- DOI: 10.1186/s13045-015-0174-9
Sampling circulating tumor cells for clinical benefits: how frequent?
Abstract
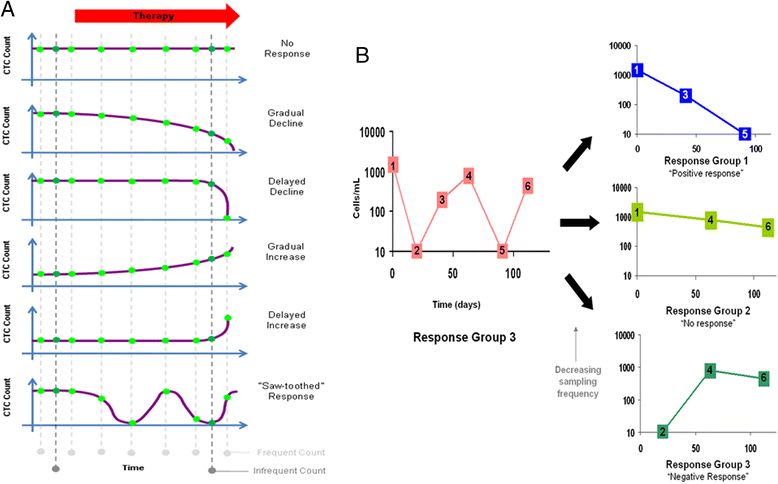
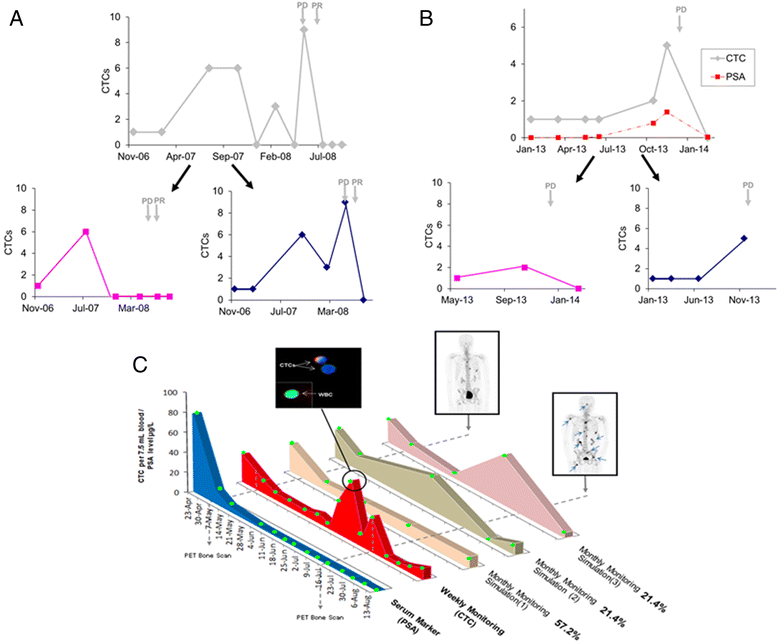
Circulating tumor cells (CTCs) are cells shed from tumors or metastatic sites and are a potential biomarker for cancer diagnosis, management, and prognostication. The majority of current studies use single or infrequent CTC sampling points. This strategy assumes that changes in CTC number, as well as phenotypic and molecular characteristics, are gradual with time. In reality, little is known today about the actual kinetics of CTC dissemination and phenotypic and molecular changes in the blood of cancer patients. Herein, we show, using clinical case studies and hypothetical simulation models, how sub-optimal CTC sampling may result in misleading observations with clinical consequences, by missing out on significant CTC spikes that occur in between sampling times. Initial studies using highly frequent CTC sampling are necessary to understand the dynamics of CTC dissemination and phenotypic and molecular changes in the blood of cancer patients. Such an improved understanding will enable an optimal, study-specific sampling frequency to be assigned to individual research studies and clinical trials and better inform practical clinical decisions on cancer management strategies for patient benefits.
Figures

References
-
- Ashworth TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aus Med J. 1869;14:146–9.
-
- Lang JM, Casavant BP, Beebe DJ. Circulating tumor cells: getting more from less. SciTransl Med. 2012;4:141ps13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

