Pharmacokinetics and Concentration-Effect Relationship of Oral LSD in Humans
- PMID: 26108222
- PMCID: PMC4772267
- DOI: 10.1093/ijnp/pyv072
Pharmacokinetics and Concentration-Effect Relationship of Oral LSD in Humans
Erratum in
-
Erratum.Int J Neuropsychopharmacol. 2016 Apr 27;19(10):pyw031. doi: 10.1093/ijnp/pyw031. Int J Neuropsychopharmacol. 2016. PMID: 27207904 Free PMC article. No abstract available.
Abstract
Background: The pharmacokinetics of oral lysergic acid diethylamide are unknown despite its common recreational use and renewed interest in its use in psychiatric research and practice.
Methods: We characterized the pharmacokinetic profile, pharmacokinetic-pharmacodynamic relationship, and urine recovery of lysergic acid diethylamide and its main metabolite after administration of a single oral dose of lysergic acid diethylamide (200 μg) in 8 male and 8 female healthy subjects.
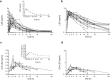
Results: Plasma lysergic acid diethylamide concentrations were quantifiable (>0.1 ng/mL) in all the subjects up to 12 hours after administration. Maximal concentrations of lysergic acid diethylamide (mean±SD: 4.5±1.4 ng/mL) were reached (median, range) 1.5 (0.5-4) hours after administration. Concentrations then decreased following first-order kinetics with a half-life of 3.6±0.9 hours up to 12 hours and slower elimination thereafter with a terminal half-life of 8.9±5.9 hours. One percent of the orally administered lysergic acid diethylamide was eliminated in urine as lysergic acid diethylamide, and 13% was eliminated as 2-oxo-3-hydroxy-lysergic acid diethylamide within 24 hours. No sex differences were observed in the pharmacokinetic profiles of lysergic acid diethylamide. The acute subjective and sympathomimetic responses to lysergic acid diethylamide lasted up to 12 hours and were closely associated with the concentrations in plasma over time and exhibited no acute tolerance.
Conclusions: These first data on the pharmacokinetics and concentration-effect relationship of oral lysergic acid diethylamide are relevant for further clinical studies and serve as a reference for the assessment of intoxication with lysergic acid diethylamide.
Keywords: LSD; O-H-LSD; pharmacodynamics; pharmacokinetics; plasma; urine.
© The Author 2015. Published by Oxford University Press on behalf of CINP.
Figures

References
-
- Abramson HA, Jarvik ME, Gorin MH, Hirsch MW. (1956) Lysergic acid diethylamide (LSD-25): XVII tolerance development and its relationship to a theory of psychosis. J Psychol 41:81–105.
-
- Aghajanian GK, Bing OH. (1964) Persistence of lysergic acid diethylamide in the plasma of human subjects. Clin Pharmacol Ther 5:611–614. - PubMed
-
- Axelrod J, Brady RO, Witkop B, Evarts EV. (1957) The distribution and metabolism of lysergic acid diethylamide. Ann N Y Acad Sci 66:435–444. - PubMed
-
- Belleville RE, Fraser HF, Isbell H, Wikler A, Logan CR. (1956) Studies on lysergic acid diethylamide (LSD-25): I. Effects in former morphine addicts and development of tolerance during chronic intoxication. AMA Arch Neurol Psychiatry 76:468–478. - PubMed
-
- Blessing WW, Seaman B. (2003) 5-hydroxytryptamine2A receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience 117:939–948. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

