A comprehensive review of the pharmacodynamics of the SGLT2 inhibitor empagliflozin in animals and humans
- PMID: 26108304
- PMCID: PMC5896322
- DOI: 10.1007/s00210-015-1134-1
A comprehensive review of the pharmacodynamics of the SGLT2 inhibitor empagliflozin in animals and humans
Abstract
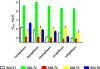
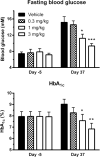
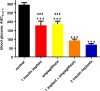
Empagliflozin (formerly known as BI 10773) is a potent, competitive, and selective inhibitor of the sodium glucose transporter SGLT2, which mediates glucose reabsorption in the early proximal tubule and most of the glucose reabsorption by the kidney, overall. Accordingly, empagliflozin treatment increased urinary glucose excretion. This has been observed across multiple species including humans and was reported under euglycemic conditions, in obesity and, most importantly, in type 2 diabetic patients and multiple animal models of type 2 diabetes and of type 1 diabetes. This led to a reduction in blood glucose, smaller blood glucose excursions during oral glucose tolerance tests, and, upon chronic treatment, a reduction in HbA1c in animal models and patients. In rodents, such effects were observed in early and late phases of experimental diabetes and were associated with preservation of pancreatic β-cell function. Combination studies in animals demonstrated that beneficial metabolic effects of empagliflozin may also manifest when added to other types of anti-hyperglycemic treatments including linagliptin and pioglitazone. While some anti-hyperglycemic drugs lead to weight gain, empagliflozin treatment was associated with reduced body weight in normoglycemic obese and non-obese animals despite an increased food intake, largely due to a loss of adipose tissue; on the other hand, empagliflozin preserved body weight in models of type 1 diabetes. Empagliflozin improved endothelial dysfunction in diabetic rats and arterial stiffness, reduced blood pressure in diabetic patients, and attenuated early signs of nephropathy in diabetic animal models. Taken together, the SGLT2 inhibitor empagliflozin improves glucose metabolism by enhancing urinary glucose excretion; upon chronic administration, at least in animal models, the reductions in blood glucose levels are associated with beneficial effects on cardiovascular and renal complications of diabetes.
Figures

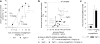


References
-
- Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32(4):515–531. - PubMed
-
- Adler AI, Steevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. - PubMed
-
- Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci. 2011;32(2):63–71. - PubMed
-
- Bonner C, Popescu I, Queniat G, Gmyr V, Moerman E, Beaucamps C, Delalleau N, Thevent J, Malasse WJ, Sener A, Abderrahmani A, Staels B, Kerr-Conte J, Pattou F. The glucose transporter SGLT2 is expressed in human pancreatic alpha cells and is required for proper control of glucagon secretion in type 2 diabetes. Diabetes. 2014;63(Suppl 1):A101.
-
- Cangoz S, Chang Y-Y, Chempakaseril SJ, Guduru RC, Huynh LM, John JS, John ST, Joseph ME, Judge R, Kimmey R, Kudratov K, Lee PJ, Madhani IC, Shim PJ, Singh S, Ruchalski C, Raffa RB. The kidney as a new target for antidiabetic drugs: SGLT2 inhibitors. J Clin Pharm Ther. 2013;38(5):350–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

