The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure
- PMID: 26108562
- PMCID: PMC4575577
- DOI: 10.1113/JP270195
The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure
Abstract
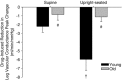
The passive leg movement (PLM) model is a novel approach to assess vascular function. Increasing femoral perfusion pressure (FPP) by moving from the supine to the upright-seated posture augments the vasodilatory response to PLM in the young, with no effect in the old, but whether this augmented vasodilatation is nitric oxide (NO) dependent is unknown. Using an intra-arterial infusion of N(G) -monomethyl-L -arginine (L -NMMA) to inhibit nitric oxide synthase (NOS), the posture-induced increases in the PLM responses in the young were nearly ablated, with no effect of NOS inhibition in the old. Therefore, PLM in combination with alterations in posture can be used to determine changes in NO-mediated vasodilatation with age, and thus, may be a clinically useful tool for assessing NO bioavailability across the human lifespan. We sought to better understand the contribution of nitric oxide (NO) to passive leg movement (PLM)-induced vasodilatation with age, with and without a posture-induced increase in femoral perfusion pressure (FPP). PLM was performed in eight young (24 ± 1 years) and eight old (74 ± 3 years) healthy males, with and without NO synthase inhibition via intra-arterial infusion of N(G) -monomethyl-L -arginine (L -NMMA) into the common femoral artery in both the supine and upright-seated posture. Central and peripheral haemodynamic responses were determined second-by-second with finger photoplethysmography and Doppler ultrasound, respectively. PLM-induced increases in heart rate, stroke volume, cardiac output and reductions in mean arterial pressure were similar between age groups and conditions. In the young, L -NMMA attenuated the peak change in leg vascular conductance (ΔLVCpeak ) in both the supine (control: 7.4 ± 0.9; L -NMMA: 5.2 ± 1.1 ml min(-1) mmHg(-1) , P < 0.05) and upright-seated (control: 12.3 ± 2.0; L -NMMA: 6.4 ± 1.0 ml min(-1) mmHg(-1) , P < 0.05) posture, with no significant change in the old (supine control: 4.2 ± 1.3; supine L -NMMA: 3.4 ± 0.8; upright-seated control: 4.5 ± 0.8; upright-seated L -NMMA: 3.4 ± 0.8 ml min(-1) mmHg(-1) , P > 0.05). Increased FPP augmented the ΔLVCpeak in the young control condition only (P < 0.05). In the upright-seated posture, NOS inhibition attenuated the FPP-induced augmentation of rapid vasodilatation in the young (control: 1.25 ± 0.23; L -NMMA: 0.74 ± 0.11 ml min(-1) mmHg(-1) s(-1) ; P < 0.05), but not the old (control: 0.37 ± 0.07; L -NMMA: 0.25 ± 0.07 ml ml min(-1) mmHg(-1) s(-1) ; P > 0.05). These data reveal that greater FPP increases the role of NO in PLM-induced vasodilatation in the young, but not the old, due to reduced NO bioavailability with age. Therefore, PLM involving alterations in posture may be useful to determine changes in NO bioavailability with age.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures



Comment in
-
A 'passive' movement into the future of assessing endothelial dysfunction?J Physiol. 2016 Mar 15;594(6):1525-6. doi: 10.1113/JP271888. J Physiol. 2016. PMID: 26995261 Free PMC article. No abstract available.
References
-
- Al-Shaer MH, Choueiri NE, Correia ML, Sinkey CA, Barenz TA. Haynes WG. Effects of aging and atherosclerosis on endothelial and vascular smooth muscle function in humans. Int J Cardiol. 2006;109:201–206. - PubMed
-
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. - PubMed
-
- Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ. Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol (1985) 1998;85:2249–2254. - PubMed
-
- Carlson RE, Kirby BS, Voyles WF. Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol. 2008;294:H1963–H1970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

