Phosphorylation of Beet black scorch virus coat protein by PKA is required for assembly and stability of virus particles
- PMID: 26108567
- PMCID: PMC4479801
- DOI: 10.1038/srep11585
Phosphorylation of Beet black scorch virus coat protein by PKA is required for assembly and stability of virus particles
Abstract
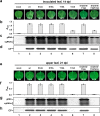
Plant virus coat proteins (CPs) play a fundamental role in protection of genomic RNAs, virion assembly, and viral movement. Although phosphorylation of several CPs during virus infection have been reported, little information is available about CP phosphorylation of the spherical RNA plant viruses. Here, we demonstrate that the CP of Beet black scorch virus (BBSV), a member of the genus Necrovirus, can be phosphorylated at threonine-41 (T41) by cAMP-dependent protein kinase (PKA)-like kinase in vivo and in vitro. Mutant viruses containing a T41A non-phosphorylatable alanine substitution, and a T41E glutamic acid substitution to mimic threonine phosphorylation were able to replicate but were unable to move systemically in Nicotiana benthamiana. Interestingly, the T41A and T41E mutants generated unstable 17 nm virus-like particles that failed to package viral genomic (g) RNA, compared with wild-type BBSV with 30 nm virions during viral infection in N. benthamiana. Further analyses showed that the T41 mutations had little effect on the gRNA-binding activity of the CP. Therefore, we propose a model whereby CP phosphorylation plays an essential role in long-distance movement of BBSV that involves formation of stable virions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Cui X. An icosahedral virus found in sugar beet. J. Shihezi Agric. College 10, 73–78 (1988).
-
- Merhvar M. & Bragard C. Beet black scorch virus in Iran is more diverse than anywhere. Phytopathol. 99, S84–S85 (2009).
-
- Weiland J. J. et al. Characterization of a US Isolate of Beet black scorch virus. Phytopathol. 97, 1245–1254 (2007). - PubMed
-
- Weiland J. J., Larson R. L., Freeman T. P. & Edwards M. C. First report of Beet black scorch virus in the United States. Plant Dis. 90, 828–828 (2006). - PubMed
-
- Koenig R. & Valizadeh J. Molecular and serological characterization of an Iranian isolate of Beet black scorch virus. Arch. Virol. 153, 1397–1400 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

