Nuclear-localized CTP:phosphocholine cytidylyltransferase α regulates phosphatidylcholine synthesis required for lipid droplet biogenesis
- PMID: 26108622
- PMCID: PMC4571330
- DOI: 10.1091/mbc.E15-03-0159
Nuclear-localized CTP:phosphocholine cytidylyltransferase α regulates phosphatidylcholine synthesis required for lipid droplet biogenesis
Abstract
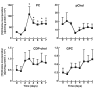
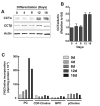
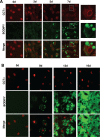
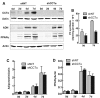
The reversible association of CTP:phosphocholine cytidylyltransferase α (CCTα) with membranes regulates the synthesis of phosphatidylcholine (PC) by the CDP-choline (Kennedy) pathway. Based on results with insect CCT homologues, translocation of nuclear CCTα onto cytoplasmic lipid droplets (LDs) is proposed to stimulate the synthesis of PC that is required for LD biogenesis and triacylglycerol (TAG) storage. We examined whether this regulatory mechanism applied to LD biogenesis in mammalian cells. During 3T3-L1 and human preadipocyte differentiation, CCTα expression and PC synthesis was induced. In 3T3-L1 cells, CCTα translocated from the nucleoplasm to the nuclear envelope and cytosol but did not associate with LDs. The enzyme also remained in the nucleus during human adipocyte differentiation. RNAi silencing in 3T3-L1 cells showed that CCTα regulated LD size but did not affect TAG storage or adipogenesis. LD biogenesis in nonadipocyte cell lines treated with oleate also promoted CCTα translocation to the nuclear envelope and/or cytoplasm but not LDs. In rat intestinal epithelial cells, CCTα silencing increased LD size, but LD number and TAG deposition were decreased due to oleate-induced cytotoxicity. We conclude that CCTα increases PC synthesis for LD biogenesis by translocation to the nuclear envelope and not cytoplasmic LDs.
© 2015 Aitchison et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



References
-
- Arnold RS, Cornell RB. Lipid regulation of CTP: phosphocholine cytidylyltransferase: electrostatic, hydrophobic, and synergistic interactions of anionic phospholipids and diacylglycerol. Biochemistry. 1996;35:9917–9924. - PubMed
-
- Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

