Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP
- PMID: 26109051
- PMCID: PMC4495399
- DOI: 10.1101/gad.264234.115
Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP
Abstract
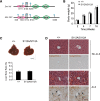
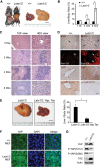
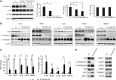
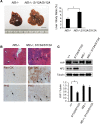
The Hippo signaling pathway converges on YAP to regulate growth, differentiation, and regeneration. Previous studies with overexpressed proteins have shown that YAP is phosphorylated by its upstream kinase, Lats1/2, on multiple sites, including an evolutionarily conserved 14-3-3-binding site whose phosphorylation is believed to inhibit YAP by excluding it from the nucleus. Indeed, nuclear localization of YAP or decreased YAP phosphorylation at this site (S168 in Drosophila, S127 in humans, and S112 in mice) is widely used in current literature as a surrogate of YAP activation even though the physiological importance of this phosphorylation event in regulating endogenous YAP activity has not been defined. Here we address this question by introducing a Yap(S112A) knock-in mutation in the endogenous Yap locus. The Yap(S112A) mice are surprisingly normal despite nuclear localization of the mutant YAP protein in vivo and profound defects in cytoplasmic translocation in vitro. Interestingly, the mutant Yap(S112A) mice show a compensatory decrease in YAP protein levels due to increased phosphorylation at a mammalian-specific phosphodegron site on YAP. These findings reveal a robust homeostatic mechanism that maintains physiological levels of YAP activity and caution against the assumptive use of YAP localization alone as a surrogate of YAP activity.
Keywords: 14-3-3; Hippo signaling; YAP oncoprotein; phosphorylation; tumorigenesis.
© 2015 Chen et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Barry ER, Camargo FD. 2013. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol 25: 247–253. - PubMed
-
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. 2007. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
