Epigenetic therapy overcomes treatment resistance in T cell prolymphocytic leukemia
- PMID: 26109102
- PMCID: PMC4807901
- DOI: 10.1126/scitranslmed.aaa5079
Epigenetic therapy overcomes treatment resistance in T cell prolymphocytic leukemia
Abstract
T cell prolymphocytic leukemia (T-PLL) is a rare, mature T cell neoplasm with distinct features and an aggressive clinical course. Early relapse and short overall survival are commonplace. Use of the monoclonal anti-CD52 antibody alemtuzumab has improved the rate of complete remission and duration of response to more than 50% and between 6 and 12 months, respectively. Despite this advance, without an allogeneic transplant, resistant relapse is inevitable. We report seven complete and one partial remission in eight patients receiving alemtuzumab and cladribine with or without a histone deacetylase inhibitor. These data show that administration of epigenetic agents can overcome alemtuzumab resistance. We also report epigenetically induced expression of the surface receptor protein CD30 in T-PLL. Subsequent treatment with the anti-CD30 antibody-drug conjugate brentuximab vedotin overcame organ-specific (skin) resistance to alemtuzumab. Our findings demonstrate activity of combination epigenetic and immunotherapy in the incurable illness T-PLL, particularly in the setting of previous alemtuzumab therapy.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

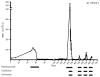


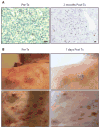

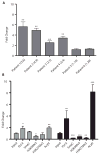
Comment in
-
Epigenetic therapy in a new era of medicine: creating and integrating molecular profiles of patients.Ann Transl Med. 2016 Nov;4(21):436. doi: 10.21037/atm.2016.11.19. Ann Transl Med. 2016. PMID: 27942527 Free PMC article. No abstract available.
References
-
- Robak T, Robak P. Current treatment options in prolymphocytic leukemia. Med Sci Monit. 2007;13:RA69–80. - PubMed
-
- Dearden CE. T-cell prolymphocytic leukemia. Med Oncol. 2006;23:17–22. - PubMed
-
- Matutes E, Brito-Babapulle V, Swansbury J, Ellis J, Morilla R, Dearden C, Sempere A, Catovsky D. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78:3269–3274. - PubMed
-
- Pawson R, Schulz TF, Matutes E, Catovsky D. The human T-cell lymphotropic viruses types I/II are not involved in T prolymphocytic leukemia and large granular lymphocytic leukemia. Leukemia. 1997;11:1305–1311. - PubMed
-
- Dearden C. How I treat prolymphocytic leukemia. Blood. 2012;120:538–551. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

