Cell migration and antigen capture are antagonistic processes coupled by myosin II in dendritic cells
- PMID: 26109323
- PMCID: PMC4491822
- DOI: 10.1038/ncomms8526
Cell migration and antigen capture are antagonistic processes coupled by myosin II in dendritic cells
Erratum in
-
Corrigendum: Cell migration and antigen capture are antagonistic processes coupled by myosin II in dendritic cells.Nat Commun. 2015 Aug 14;6:8122. doi: 10.1038/ncomms9122. Nat Commun. 2015. PMID: 26271903 Free PMC article. No abstract available.
Abstract
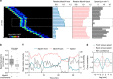
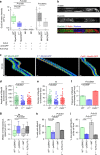
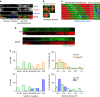
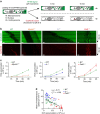
The immune response relies on the migration of leukocytes and on their ability to stop in precise anatomical locations to fulfil their task. How leukocyte migration and function are coordinated is unknown. Here we show that in immature dendritic cells, which patrol their environment by engulfing extracellular material, cell migration and antigen capture are antagonistic. This antagonism results from transient enrichment of myosin IIA at the cell front, which disrupts the back-to-front gradient of the motor protein, slowing down locomotion but promoting antigen capture. We further highlight that myosin IIA enrichment at the cell front requires the MHC class II-associated invariant chain (Ii). Thus, by controlling myosin IIA localization, Ii imposes on dendritic cells an intermittent antigen capture behaviour that might facilitate environment patrolling. We propose that the requirement for myosin II in both cell migration and specific cell functions may provide a general mechanism for their coordination in time and space.
Figures




References
-
- Sallusto F., Cella M., Danieli C. & Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400 (1995). - PMC - PubMed
-
- Garrett W. S. et al.. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102, 325–334 (2000). - PubMed
-
- West M. A., Prescott A. R., Eskelinen E. L., Ridley A. J. & Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr. Biol. 10, 839–848 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

