Primase-polymerases are a functionally diverse superfamily of replication and repair enzymes
- PMID: 26109351
- PMCID: PMC4538821
- DOI: 10.1093/nar/gkv625
Primase-polymerases are a functionally diverse superfamily of replication and repair enzymes
Abstract
Until relatively recently, DNA primases were viewed simply as a class of proteins that synthesize short RNA primers requisite for the initiation of DNA replication. However, recent studies have shown that this perception of the limited activities associated with these diverse enzymes can no longer be justified. Numerous examples can now be cited demonstrating how the term 'DNA primase' only describes a very narrow subset of these nucleotidyltransferases, with the vast majority fulfilling multifunctional roles from DNA replication to damage tolerance and repair. This article focuses on the archaeo-eukaryotic primase (AEP) superfamily, drawing on recently characterized examples from all domains of life to highlight the functionally diverse pathways in which these enzymes are employed. The broad origins, functionalities and enzymatic capabilities of AEPs emphasizes their previous functional misannotation and supports the necessity for a reclassification of these enzymes under a category called primase-polymerases within the wider functional grouping of polymerases. Importantly, the repositioning of AEPs in this way better recognizes their broader roles in DNA metabolism and encourages the discovery of additional functions for these enzymes, aside from those highlighted here.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

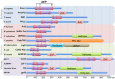



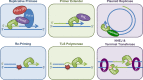
References
-
- Scherzinger E., Lanka E., Morelli G., Seiffert D., Yuki A. Bacteriophage-T7-Induced DNA-priming protein. Eur. J. Biochem. 1977;72:543–558. - PubMed
-
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J. Biol. Chem. 1978;253:758–764. - PubMed
-
- Bouché J.P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J. Biol. Chem. 1975;250:5995–6001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

