Optimal drug regimens for primary biliary cirrhosis: a systematic review and network meta-analysis
- PMID: 26109432
- PMCID: PMC4695204
- DOI: 10.18632/oncotarget.4528
Optimal drug regimens for primary biliary cirrhosis: a systematic review and network meta-analysis
Abstract
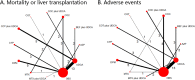
Objective: Most comprehensive treatments for PBC include UDCA, combination of methotrexate (MTX), corticosteroids (COT), colchicine (COC) or bezafibrate (BEF), cyclosporin A (CYP), D-penicillamine (DPM), methotrexate (MTX), or azathioprine (AZP). Since the optimum treatment regimen remains inconclusive, we aimed to compare these therapies in terms of patient mortality or liver transplantation (MOLT) and adverse event (AE).
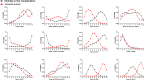
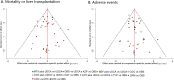
Methods: We searched PubMed, Embase, Scopus and the Cochrane Library for randomized controlled trials until August 2014. We estimated HRs for MOLT and ORs for AE. The sensitivity analysis based on dose of UDCA was also performed.
Results: The search identified 49 studies involving 12 different treatment regimens and 4182 patients. Although no statistical significance can be found in MOLT, COT plus UDCA was ranked highest for efficacy outcome amongst all the treatment regimes. While for AEs, compared with OBS or UDCA, monotherapy with COC (OR 5.6, P < 0.001; OR 5.89, P < 0.001), CYP (OR 3.24, P < 0.001; OR 3.42, P < 0.001), DPM (OR 8.00, P < 0.001; OR 8.45, P < 0.001) and MTX (OR 5.31, P < 0.001; OR 5.61, P < 0.001) were associated with statistically significant increased risk of AEs. No significant differences were found for other combination regimes. Effect estimates from indirect comparisons matched closely to estimates derived from pairwise comparisons. Consistently, in the sensitivity analysis, results closely resembled our primary analysis.
Conclusions: COT plus UDCA was the most efficacious among treatment regimens both for MOLT and AEs.
Keywords: UDCA-based therapy; adverse events; indirect comparison; network meta-analysis; primary biliary cirrhosis.
Conflict of interest statement
The authors report no declarations of interest.
Figures

References
-
- Kaplan MM, Cheng S, Price LL, Bonis PA. A randomized controlled trial of colchicine plus ursodiol versus methotrexate plus ursodiol in primary biliary cirrhosis: ten-year results. Hepatology (Baltimore, Md.) 2004;39:915–923. - PubMed
-
- Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, Michieletti P, Minuk GY, Pappas SC, Scully LJ, et al. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology (Baltimore, Md.) 1994;19:1149–1156. - PubMed
-
- Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Harrison JM, Wiesner RH, Anderson ML, Lange SM, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–1290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

