Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: a review
- PMID: 26109634
- PMCID: PMC4528590
- DOI: 10.1098/rsif.2015.0254
Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: a review
Abstract
The development of biomaterials for cardiac tissue engineering (CTE) is challenging, primarily owing to the requirement of achieving a surface with favourable characteristics that enhances cell attachment and maturation. The biomaterial surface plays a crucial role as it forms the interface between the scaffold (or cardiac patch) and the cells. In the field of CTE, synthetic polymers (polyglycerol sebacate, polyethylene glycol, polyglycolic acid, poly-l-lactide, polyvinyl alcohol, polycaprolactone, polyurethanes and poly(N-isopropylacrylamide)) have been proven to exhibit suitable biodegradable and mechanical properties. Despite the fact that they show the required biocompatible behaviour, most synthetic polymers exhibit poor cell attachment capability. These synthetic polymers are mostly hydrophobic and lack cell recognition sites, limiting their application. Therefore, biofunctionalization of these biomaterials to enhance cell attachment and cell material interaction is being widely investigated. There are numerous approaches for functionalizing a material, which can be classified as mechanical, physical, chemical and biological. In this review, recent studies reported in the literature to functionalize scaffolds in the context of CTE, are discussed. Surface, morphological, chemical and biological modifications are introduced and the results of novel promising strategies and techniques are discussed.
Figures

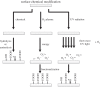

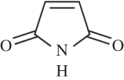
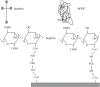


References
-
- Improvement P, Goldberg LR. 2010. Heart failure. Ann. Intern. Med. 152, ITC61–15; quiz ITC616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
