Bacterial Membrane Vesicles Mediate the Release of Mycobacterium tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages
- PMID: 26109643
- PMCID: PMC4506856
- DOI: 10.4049/jimmunol.1402894
Bacterial Membrane Vesicles Mediate the Release of Mycobacterium tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages
Abstract
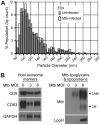
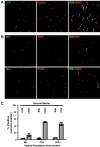
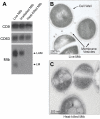
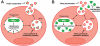
Mycobacterium tuberculosis is an intracellular pathogen that infects lung macrophages and releases microbial factors that regulate host defense. M. tuberculosis lipoproteins and lipoglycans block phagosome maturation, inhibit class II MHC Ag presentation, and modulate TLR2-dependent cytokine production, but the mechanisms for their release during infection are poorly defined. Furthermore, these molecules are thought to be incorporated into host membranes and released from infected macrophages within exosomes, 40-150-nm extracellular vesicles that derive from multivesicular endosomes. However, our studies revealed that extracellular vesicles released from infected macrophages include two distinct, largely nonoverlapping populations: one containing host cell markers of exosomes (CD9, CD63) and the other containing M. tuberculosis molecules (lipoglycans, lipoproteins). These vesicle populations are similar in size but have distinct densities, as determined by separation on sucrose gradients. Release of lipoglycans and lipoproteins from infected macrophages was dependent on bacterial viability, implicating active bacterial mechanisms in their secretion. Consistent with recent reports of extracellular vesicle production by bacteria (including M. tuberculosis), we propose that bacterial membrane vesicles are secreted by M. tuberculosis within infected macrophages and subsequently are released into the extracellular environment. Furthermore, extracellular vesicles released from M. tuberculosis-infected cells activate TLR2 and induce cytokine responses by uninfected macrophages. We demonstrate that these activities derive from the bacterial membrane vesicles rather than exosomes. Our findings suggest that bacterial membrane vesicles are the primary means by which M. tuberculosis exports lipoglycans and lipoproteins to impair effector functions of infected macrophages and circulate bacterial components beyond the site of infection to regulate immune responses by uninfected cells.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures



References
-
- Pennini ME, Pai RK, Schultz DC, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J. Immunol. 2006;176:4323–4330. - PubMed
-
- Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19 kD lipoprotein of Mycobacterium tuberculosis. J. Immunol. 2001;167:910–918. - PubMed
-
- Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 2003;171:175–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

