Superoxide-Generating Nox5α Is Functionally Required for the Human T-Cell Leukemia Virus Type 1-Induced Cell Transformation Phenotype
- PMID: 26109726
- PMCID: PMC4524067
- DOI: 10.1128/JVI.00983-15
Superoxide-Generating Nox5α Is Functionally Required for the Human T-Cell Leukemia Virus Type 1-Induced Cell Transformation Phenotype
Abstract
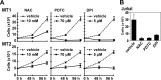
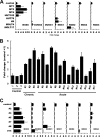
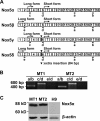
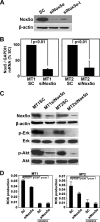
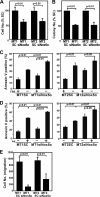
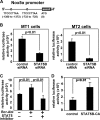
Human T-cell leukemia virus type 1 (HTLV-1) is associated with adult T-cell leukemia (ATL) and transforms T cells in vitro. To our knowledge, the functional role of reactive oxygen species (ROS)-generating NADPH oxidase 5 (Nox5) in HTLV-1 transformation remains undefined. Here, we found that Nox5α expression was upregulated in 88% of 17 ATL patient samples but not in normal peripheral blood T cells. Upregulation of the Nox5α variant was transcriptionally sustained by the constitutive Janus family tyrosine kinase (Jak)-STAT5 signaling pathway in interleukin-2 (IL-2)-independent HTLV-1-transformed cell lines, including MT1 and MT2, whereas it was transiently induced by the IL-2-triggered Jak-STAT5 axis in uninfected T cells. A Nox inhibitor, diphenylene iodonium, and antioxidants such as N-acetyl cysteine blocked proliferation of MT1 and MT2 cells. Ablation of Nox5α by small interfering RNAs abrogated ROS production, inhibited cellular activities, including proliferation, migration, and survival, and suppressed tumorigenicity in immunodeficient NOG mice. The findings suggest that Nox5α is a key molecule for redox-signal-mediated maintenance of the HTLV-1 transformation phenotype and could be a potential molecular target for therapeutic intervention in cancer development.
Importance: HTLV-1 is the first human oncogenic retrovirus shown to be associated with ATL. Despite the extensive study over the years, the mechanism underlying HTLV-1-induced cell transformation is not fully understood. In this study, we addressed the expression and function of ROS-generating Nox family genes in HTLV-1-transformed cells. Our report provides the first evidence that the upregulated expression of Nox5α is associated with the pathological state of ATL peripheral blood mononuclear cells and that Nox5α is an integral component of the Jak-STAT5 signaling pathway in HTLV-1-transformed T cells. Nox5α-derived ROS are critically involved in the regulation of cellular activities, including proliferation, migration, survival, and tumorigenicity, in HTLV-1-transformed cells. These results indicate that Nox5α-derived ROS are functionally required for maintenance of the HTLV-1 transformation phenotype. The finding provides new insight into the redox-dependent mechanism of HTLV-1 transformation and raises an intriguing possibility that Nox5α serves as a potential molecular target to treat HTLV-1-related leukemia.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Functional uncoupling of the Janus kinase 3-Stat5 pathway in malignant growth of human T cell leukemia virus type 1-transformed human T cells.J Immunol. 2000 Nov 1;165(9):5097-104. doi: 10.4049/jimmunol.165.9.5097. J Immunol. 2000. PMID: 11046040
-
Down-regulation of SHP1 and up-regulation of negative regulators of JAK/STAT signaling in HTLV-1 transformed cell lines and freshly transformed human peripheral blood CD4+ T-cells.Leuk Res. 2004 Jan;28(1):71-82. doi: 10.1016/s0145-2126(03)00158-9. Leuk Res. 2004. PMID: 14630083
-
Activation of AID by human T-cell leukemia virus Tax oncoprotein and the possible role of its constitutive expression in ATL genesis.Carcinogenesis. 2011 Jan;32(1):110-9. doi: 10.1093/carcin/bgq222. Epub 2010 Oct 25. Carcinogenesis. 2011. PMID: 20974684
-
Research and discovery of the first human cancer virus, HTLV-1.Best Pract Res Clin Haematol. 2011 Dec;24(4):559-65. doi: 10.1016/j.beha.2011.09.012. Epub 2011 Nov 16. Best Pract Res Clin Haematol. 2011. PMID: 22127321 Review.
-
Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation.Nat Rev Cancer. 2007 Apr;7(4):270-80. doi: 10.1038/nrc2111. Nat Rev Cancer. 2007. PMID: 17384582 Review.
Cited by
-
NADPH oxidase 5α promotes the formation of CD271 tumor-initiating cells in oral cancer.Am J Cancer Res. 2020 Jun 1;10(6):1710-1727. eCollection 2020. Am J Cancer Res. 2020. PMID: 32642285 Free PMC article.
-
The Redox-Metabolic Couple of T Lymphocytes: Potential Consequences for Hypertension.Antioxid Redox Signal. 2021 Apr 20;34(12):915-935. doi: 10.1089/ars.2020.8042. Epub 2020 Apr 30. Antioxid Redox Signal. 2021. PMID: 32237890 Free PMC article. Review.
-
Carcinogenesis and Reactive Oxygen Species Signaling: Interaction of the NADPH Oxidase NOX1-5 and Superoxide Dismutase 1-3 Signal Transduction Pathways.Antioxid Redox Signal. 2019 Jan 20;30(3):443-486. doi: 10.1089/ars.2017.7268. Epub 2018 Nov 22. Antioxid Redox Signal. 2019. PMID: 29478325 Free PMC article. Review.
-
Inhibiting the Activity of NADPH Oxidase in Cancer.Antioxid Redox Signal. 2020 Aug 20;33(6):435-454. doi: 10.1089/ars.2020.8046. Epub 2020 Apr 17. Antioxid Redox Signal. 2020. PMID: 32008376 Free PMC article.
-
Reactive Oxygen Species and Metabolism in Leukemia: A Dangerous Liaison.Front Immunol. 2022 Jun 9;13:889875. doi: 10.3389/fimmu.2022.889875. eCollection 2022. Front Immunol. 2022. PMID: 35757686 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

