Patient-Specific Neutralizing Antibody Responses to Herpes Simplex Virus Are Attributed to Epitopes on gD, gB, or Both and Can Be Type Specific
- PMID: 26109729
- PMCID: PMC4542347
- DOI: 10.1128/JVI.01213-15
Patient-Specific Neutralizing Antibody Responses to Herpes Simplex Virus Are Attributed to Epitopes on gD, gB, or Both and Can Be Type Specific
Abstract
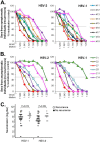
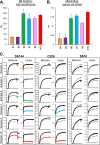
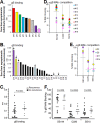
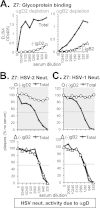
Herpes simplex virus 1 (HSV-1) and HSV-2 infect many humans and establish a latent infection in sensory ganglia. Although some infected people suffer periodic recurrences, others do not. Infected people mount both cell-mediated and humoral responses, including the production of virus-neutralizing antibodies (Abs) directed at viral entry glycoproteins. Previously, we examined IgGs from 10 HSV-seropositive individuals; all neutralized virus and were directed primarily against gD or gD+gB. Here, we expand our studies and examine 32 additional sera from HSV-infected individuals, 23 of whom had no recurrent disease. Using an Octet RED96 system, we screened all 32 serum samples directly for both glycoprotein binding and competition with known neutralizing anti-gD and -gB monoclonal Abs (MAbs). On average, the recurrent cohort exhibited higher binding to gD and gB and had higher neutralization titers. There were similar trends in the blocking of MAbs to critical gD and gB epitopes. When we depleted six sera of Abs to specific glycoproteins, we found different types of responses, but always directed primarily at gD and/or gB. Interestingly, in one dual-infected person, the neutralizing response to HSV-2 was due to gD2 and gB2, whereas HSV-1 neutralization was due to gD1 and gB1. In another case, virus neutralization was HSV-1 specific, with the Ab response directed entirely at gB1, despite this serum blocking type-common anti-gD and -gB neutralizing MAbs. These data are pertinent in the design of future HSV vaccines since they demonstrate the importance of both serotypes of gD and gB as immunogens.
Importance: We previously showed that people infected with HSV produce neutralizing Abs directed against gD or a combination of gD+gB (and in one case, gD+gB+gC, which was HSV-1 specific). In this more extensive study, we again found that gD or gD+gB can account for the virus neutralizing response and critical epitopes of one or both of these proteins are represented in sera of naturally infected humans. However, we also found that some individuals produced a strong response against gB alone. In addition, we identified type-specific contributions to HSV neutralization from both gD and gB. Contributions from the other entry glycoproteins, gC and gH/gL, were minimal and limited to HSV-1 neutralization. Knowing the variations in how humans see and mount a response to HSV will be important to vaccine development.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

