The Interferon-Inducible Protein Tetherin Inhibits Hepatitis B Virus Virion Secretion
- PMID: 26109732
- PMCID: PMC4542364
- DOI: 10.1128/JVI.00933-15
The Interferon-Inducible Protein Tetherin Inhibits Hepatitis B Virus Virion Secretion
Abstract
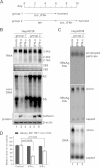
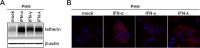
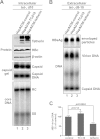
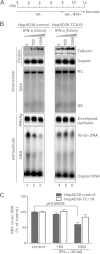
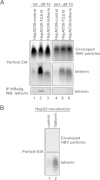
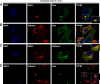
Interferon alpha (IFN-α) is an approved medication for chronic hepatitis B therapy. Besides acting as an immunomodulator, IFN-α elicits a pleiotropic antiviral state in hepatitis B virus (HBV)-infected hepatocytes, but whether or not IFN-α impedes the late steps of the HBV life cycle, such as HBV secretion, remains elusive. Here we report that IFN-α treatment of HepAD38 cells with established HBV replication selectively reduced HBV virion release without altering intracellular viral replication or the secretion of HBV subviral particles and nonenveloped capsids. In search of the interferon-stimulated gene(s) that is responsible for the reduction of HBV virion release, we found that tetherin, a broad-spectrum antiviral transmembrane protein that inhibits the egress of a variety of enveloped viruses, was highly induced by IFN-α in HepAD38 cells and in primary human hepatocytes. We further demonstrated that the expression of full-length tetherin, but not the C-terminal glycosylphosphatidylinositol (GPI) anchor-truncated form, inhibited HBV virion egress from HepAD38 cells. In addition, GPI anchor-truncated tetherin exhibited a dominant-negative effect and was incorporated into the liberated virions. We also found colocalization of tetherin and HBV L protein at the intracellular multivesicular body, where the budding of HBV virions takes place. In line with this, electron microscopy demonstrated that HBV virions were tethered in the lumen of the cisterna membrane under tetherin expression. Finally, knockdown of tetherin or overexpression of dominant negative tetherin attenuated the IFN-α-mediated reduction of HBV virion release. Taken together, our study suggests that IFN-α inhibits HBV virion egress from hepatocytes through the induction of tetherin.
Importance: Tetherin is a host restriction factor that blocks the egress of a variety of enveloped viruses through tethering the budding virions on the cell surface with its membrane anchor domains. Here we report that interferon directly and selectively inhibits the secretion of HBV virions, but not subviral particles or nonenveloped capsids, through the induction of tetherin in hepatocyte-derived cells. The antiviral function of tetherin requires the carboxyl-terminal GPI anchor, while the GPI anchor deletion mutant exhibits dominant negative activity and attaches to liberated HBV virions. Consistent with the fact that HBV is an intracellular budding virus, microscopy analyses demonstrated that the tethering of HBV virions occurs in the intracellular cisterna and that tetherin colocalizes with HBV virions on the multivesicular body, which is the HBV virion budding site. Our study not only expands the antiviral spectrum of tetherin but also sheds light on the mechanisms of interferon-elicited anti-HBV responses.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

