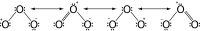Ozone decomposition
- PMID: 26109880
- PMCID: PMC4427716
- DOI: 10.2478/intox-2014-0008
Ozone decomposition
Abstract
Catalytic ozone decomposition is of great significance because ozone is a toxic substance commonly found or generated in human environments (aircraft cabins, offices with photocopiers, laser printers, sterilizers). Considerable work has been done on ozone decomposition reported in the literature. This review provides a comprehensive summary of the literature, concentrating on analysis of the physico-chemical properties, synthesis and catalytic decomposition of ozone. This is supplemented by a review on kinetics and catalyst characterization which ties together the previously reported results. Noble metals and oxides of transition metals have been found to be the most active substances for ozone decomposition. The high price of precious metals stimulated the use of metal oxide catalysts and particularly the catalysts based on manganese oxide. It has been determined that the kinetics of ozone decomposition is of first order importance. A mechanism of the reaction of catalytic ozone decomposition is discussed, based on detailed spectroscopic investigations of the catalytic surface, showing the existence of peroxide and superoxide surface intermediates.
Keywords: catalysts; decomposition; kinetics; mechanism; ozone; synthesis.
Figures
References
-
- Aktyacheva L, Emelyanova G. Activated carbon treatment with ozone. J Phys Chem. 1990;31:21. [in Rus]
-
- Alexandrov YA, Tarunin BI, Perepletchikov ML. UV absorption of gaseous ozone. J Phys Chem. 1983;LVII(10):2385–2397. [in Rus]
-
- Atale S, Hitoshi M, Kaneko N, Taraichi K, Yano M. Ozone reactions with various carbon materials. Jap Pat CA. 1995;123:121871.
-
- Baldi M, Finochhio E, Pistarino C, Busca G. Evaluation of the mechanism of the oxy-dehydrogenation of propane over manganese oxide. J Appl Catal A: General. 1998;173:61–74.
-
- Baltanas MA, Stiles AB, Katzer JR. Development of supported manganese oxides catalysts for partial oxidation: Preparation and hydrogenation properties. Appl Catal. 1986;28:13–33.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources