Protocols and Hospital Mortality in Critically Ill Patients: The United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study
- PMID: 26110488
- PMCID: PMC5673100
- DOI: 10.1097/CCM.0000000000001157
Protocols and Hospital Mortality in Critically Ill Patients: The United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study
Abstract
Objective: Clinical protocols may decrease unnecessary variation in care and improve compliance with desirable therapies. We evaluated whether highly protocolized ICUs have superior patient outcomes compared with less highly protocolized ICUs.
Design: Observational study in which participating ICUs completed a general assessment and enrolled new patients 1 day each week.
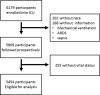
Patients: A total of 6,179 critically ill patients.
Setting: Fifty-nine ICUs in the United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study.
Interventions: None.
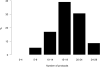
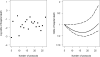
Measurements and main results: The primary exposure was the number of ICU protocols; the primary outcome was hospital mortality. A total of 5,809 participants were followed prospectively, and 5,454 patients in 57 ICUs had complete outcome data. The median number of protocols per ICU was 19 (interquartile range, 15-21.5). In single-variable analyses, there were no differences in ICU and hospital mortality, length of stay, use of mechanical ventilation, vasopressors, or continuous sedation among individuals in ICUs with a high versus low number of protocols. The lack of association was confirmed in adjusted multivariable analysis (p = 0.70). Protocol compliance with two ventilator management protocols was moderate and did not differ between ICUs with high versus low numbers of protocols for lung protective ventilation in acute respiratory distress syndrome (47% vs 52%; p = 0.28) and for spontaneous breathing trials (55% vs 51%; p = 0.27).
Conclusions: Clinical protocols are highly prevalent in U.S. ICUs. The presence of a greater number of protocols was not associated with protocol compliance or patient mortality.
Figures
References
-
- Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303. - PubMed
-
- Morris AH. Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med 7. 2000;132(5):373–383. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 HL123031/HL/NHLBI NIH HHS/United States
- P50 AA013757/AA/NIAAA NIH HHS/United States
- U01 HL108712/HL/NHLBI NIH HHS/United States
- K23 AG034257/AG/NIA NIH HHS/United States
- R01 FD003440/FD/FDA HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- R00 HL096955/HL/NHLBI NIH HHS/United States
- HL123031/HL/NHLBI NIH HHS/United States
- AG034257/AG/NIA NIH HHS/United States
- K23 GM071399/GM/NIGMS NIH HHS/United States
- K23GM094465/GM/NIGMS NIH HHS/United States
- I01 BX001786/BX/BLRD VA/United States
- K23 GM094465/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical