PINK1 Is Dispensable for Mitochondrial Recruitment of Parkin and Activation of Mitophagy in Cardiac Myocytes
- PMID: 26110811
- PMCID: PMC4482400
- DOI: 10.1371/journal.pone.0130707
PINK1 Is Dispensable for Mitochondrial Recruitment of Parkin and Activation of Mitophagy in Cardiac Myocytes
Abstract
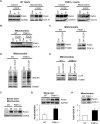
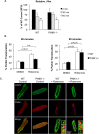
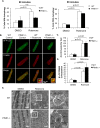
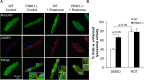
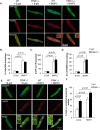
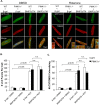
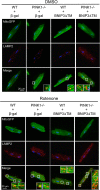
Myocyte function and survival relies on the maintenance of a healthy population of mitochondria. The PINK1/Parkin pathway plays an important role in clearing defective mitochondria via autophagy in cells. However, how the PINK1/Parkin pathway regulates mitochondrial quality control and whether it coordinates with other mitophagy pathways are still unclear. Therefore, the objective of this study was to investigate the effect of PINK1-deficiency on mitochondrial quality control in myocytes. Using PINK1-deficient (PINK1-/-) mice, we found that Parkin is recruited to damaged cardiac mitochondria in hearts after treatment with the mitochondrial uncoupler FCCP or after a myocardial infarction even in the absence of PINK1. Parkin recruitment to depolarized mitochondria correlates with increased ubiquitination of mitochondrial proteins and activation of mitophagy in PINK1-/- myocytes. In addition, induction of mitophagy by the atypical BH3-only protein BNIP3 is unaffected by lack of PINK1. Overall, these data suggest that Parkin recruitment to depolarized cardiac mitochondria and subsequent activation of mitophagy is independent of PINK1. Moreover, alternative mechanisms of Parkin activation and pathways of mitophagy remain functional in PINK1-/- myocytes and could compensate for the PINK1 deficiency.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

