Systematic review and meta-analysis of immunohistochemical prognostic biomarkers in resected oesophageal adenocarcinoma
- PMID: 26110972
- PMCID: PMC4647536
- DOI: 10.1038/bjc.2015.179
Systematic review and meta-analysis of immunohistochemical prognostic biomarkers in resected oesophageal adenocarcinoma
Erratum in
-
Systematic review and meta-analysis of immunohistochemical prognostic biomarkers in resected oesophageal adenocarcinoma.Br J Cancer. 2015 Dec 22;113(12):1746. doi: 10.1038/bjc.2015.460. Br J Cancer. 2015. PMID: 26695557 Free PMC article. No abstract available.
Abstract
Background: Oesophageal adenocarcinoma (OAC) is one of the fastest rising malignancies with continued poor prognosis. Many studies have proposed novel biomarkers but, to date, no immunohistochemical markers of survival after oesophageal resection have entered clinical practice. Here, we systematically review and meta-analyse the published literature, to identify potential biomarkers.
Methods: Relevant articles were identified via Ovid medline 1946-2013. For inclusion, studies had to conform to REporting recommendations for tumor MARKer (REMARK) prognostic study criteria. The primary end-point was a pooled hazard ratio (HR) and variance, summarising the effect of marker expression on prognosis.
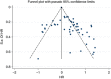
Results: A total of 3059 articles were identified. After exclusion of irrelevant titles and abstracts, 214 articles were reviewed in full. Nine molecules had been examined in more than one study (CD3, CD8, COX-2, EGFR, HER2, Ki67, LgR5, p53 and VEGF) and were meta-analysed. Markers with largest survival effects were COX-2 (HR=2.47, confidence interval (CI)=1.15-3.79), CD3 (HR=0.51, 95% CI=0.32-0.70), CD8 (HR=0.55, CI=0.31-0.80) and EGFR (HR=1.65, 95% CI=1.14-2.16).
Discussion: Current methods have not delivered clinically useful molecular prognostic biomarkers in OAC. We have highlighted the paucity of good-quality robust studies in this field. A genome-to-protein approach would be better suited for the development and subsequent validation of biomarkers. Large collaborative projects with standardised methodology will be required to generate clinically useful biomarkers.
Figures



References
-
- Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna W, Lickley L, Wilkinson R, Qizilbash A, Ambus U, Lipa M, Weizel H, Katz A, Baida M, Mariz S, Stoik G, Dacamara P, Strongitharm D, Geddie W, McCready D (1998) neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol 16(4): 1340–1349. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK To GATI (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742): 687–697. - PubMed
-
- Becker L, Huang Q, Mashimo H (2010) Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett's esophagus and esophageal adenocarcinoma. Dis Esophagus 23(2): 168–174. - PubMed
-
- Bettstetter M, Berezowska S, Keller G, Walch A, Feuchtinger A, Slotta-Huspenina J, Feith M, Drecoll E, Hofler H, Langer R (2013) Epidermal growth factor receptor, phosphatidylinositol-3-kinase catalytic subunit/PTEN, and KRAS/NRAS/BRAF in primary resected esophageal adenocarcinomas: loss of PTEN is associated with worse clinical outcome. Hum Pathol 44(5): 829–836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

