Carbocations and the Complex Flavor and Bouquet of Wine: Mechanistic Aspects of Terpene Biosynthesis in Wine Grapes
- PMID: 26111168
- PMCID: PMC6272345
- DOI: 10.3390/molecules200610781
Carbocations and the Complex Flavor and Bouquet of Wine: Mechanistic Aspects of Terpene Biosynthesis in Wine Grapes
Abstract
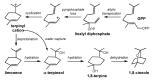
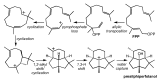
Computational chemistry approaches for studying the formation of terpenes/terpenoids in wines are presented, using five particular terpenes/terpenoids (1,8-cineole, α-ylangene, botrydial, rotundone, and the wine lactone), volatile compounds (or their precursors) found in wine and/or wine grapes, as representative examples. Through these examples, we show how modern computational quantum chemistry can be employed as an effective tool for assessing the validity of proposed mechanisms for terpene/terpenoid formation.
Keywords: aroma; biosynthesis; mechanism; quantum chemistry; terpene; wine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Terpene evolution during the development of Vitis vinifera L. cv. Shiraz grapes.Food Chem. 2016 Aug 1;204:463-474. doi: 10.1016/j.foodchem.2016.02.125. Epub 2016 Feb 22. Food Chem. 2016. PMID: 26988525
-
Terpene compounds as possible precursors of 1,8-cineole in red grapes and wines.J Agric Food Chem. 2005 Mar 9;53(5):1633-6. doi: 10.1021/jf040332d. J Agric Food Chem. 2005. PMID: 15740051
-
Aroma Precursors in Grapes and Wine: Flavor Release during Wine Production and Consumption.J Agric Food Chem. 2018 Mar 14;66(10):2281-2286. doi: 10.1021/acs.jafc.6b05255. Epub 2017 Mar 8. J Agric Food Chem. 2018. PMID: 28220693 Review.
-
Understanding the Constitutive and Induced Biosynthesis of Mono- and Sesquiterpenes in Grapes (Vitis vinifera): A Key to Unlocking the Biochemical Secrets of Unique Grape Aroma Profiles.J Agric Food Chem. 2015 Dec 16;63(49):10591-603. doi: 10.1021/acs.jafc.5b04398. Epub 2015 Dec 3. J Agric Food Chem. 2015. PMID: 26592256 Review.
-
Free terpene evolution during the berry maturation of five Vitis vinifera L. cultivars.Food Chem. 2019 Nov 30;299:125101. doi: 10.1016/j.foodchem.2019.125101. Epub 2019 Jun 29. Food Chem. 2019. PMID: 31323442
Cited by
-
Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine.Front Chem. 2018 Mar 19;6:66. doi: 10.3389/fchem.2018.00066. eCollection 2018. Front Chem. 2018. PMID: 29616214 Free PMC article.
-
Linking Terpene Synthases to Sesquiterpene Metabolism in Grapevine Flowers.Front Plant Sci. 2019 Feb 21;10:177. doi: 10.3389/fpls.2019.00177. eCollection 2019. Front Plant Sci. 2019. PMID: 30846994 Free PMC article.
-
Effects of Non-Saccharomyces Yeasts and Their Pairwise Combinations in Co-Fermentation with Saccharomyces cerevisiae on the Quality of Chunjian Citrus Wine.Molecules. 2024 Feb 27;29(5):1028. doi: 10.3390/molecules29051028. Molecules. 2024. PMID: 38474538 Free PMC article.
-
Typical Aroma of Merlot Dry Red Wine from Eastern Foothill of Helan Mountain in Ningxia, China.Molecules. 2023 Jul 27;28(15):5682. doi: 10.3390/molecules28155682. Molecules. 2023. PMID: 37570652 Free PMC article.
-
Comparative (Within Species) Genomics of the Vitis vinifera L. Terpene Synthase Family to Explore the Impact of Genotypic Variation Using Phased Diploid Genomes.Front Genet. 2020 May 5;11:421. doi: 10.3389/fgene.2020.00421. eCollection 2020. Front Genet. 2020. PMID: 32431727 Free PMC article.
References
-
- Cane D.E. Isoprenoids Including Carotenoids and Steroids. Compr. Nat. Prod. Chem. 1999;2:155–200.
-
- Cane D.E. Enzymic formation of sesquiterpenes. Chem. Rev. 1990;90:1089–1103. doi: 10.1021/cr00105a002. - DOI
-
- Davis E., Croteau R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. In: Leeper F., Vederas J., editors. Biosynthesis. Volume 209. Springer; Berlin/Heidelberg, Germany: 2000. pp. 53–95.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

