Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity
- PMID: 26111177
- PMCID: PMC6272240
- DOI: 10.3390/molecules200611474
Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity
Abstract
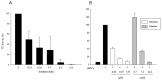
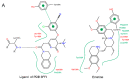
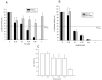
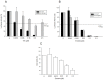
Ipecac alkaloids are secondary metabolites produced in the medicinal plant Psychotria ipecacuanha. Emetine is the main alkaloid of ipecac and one of the active compounds in syrup of Ipecac with emetic property. Here we evaluated emetine's potential as an antiviral agent against Human Immunodeficiency Virus. We performed in vitro Reverse Transcriptase (RT) Assay and Natural Endogenous Reverse Transcriptase Activity Assay (NERT) to evaluate HIV RT inhibition. Emetine molecular docking on HIV-1 RT was also analyzed. Phenotypic assays were performed in non-lymphocytic and in Peripheral Blood Mononuclear Cells (PBMC) with HIV-1 wild-type and HIV-harboring RT-resistant mutation to Nucleoside Reverse Transcriptase Inhibitors (M184V). Our results showed that HIV-1 RT was blocked in the presence of emetine in both models: in vitro reactions with isolated HIV-1 RT and intravirion, measured by NERT. Emetine revealed a strong potential of inhibiting HIV-1 replication in both cellular models, reaching 80% of reduction in HIV-1 infection, with low cytotoxic effect. Emetine also blocked HIV-1 infection of RT M184V mutant. These results suggest that emetine is able to penetrate in intact HIV particles, and bind and block reverse transcription reaction, suggesting that it can be used as anti-HIV microbicide. Taken together, our findings provide additional pharmacological information on the potential therapeutic effects of emetine.
Keywords: HIV-1; PBMC; Reverse Transcriptase; emetine; resistance.
Conflict of interest statement
The authors declared no conflict of interest.
Figures

References
-
- World Health Organization; Geneva, Switzerland: 2014. The gap report 2014: People living with HIV.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

