Instrumental Role of Helicobacter pylori γ-Glutamyl Transpeptidase in VacA-Dependent Vacuolation in Gastric Epithelial Cells
- PMID: 26111186
- PMCID: PMC4482420
- DOI: 10.1371/journal.pone.0131460
Instrumental Role of Helicobacter pylori γ-Glutamyl Transpeptidase in VacA-Dependent Vacuolation in Gastric Epithelial Cells
Abstract
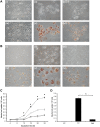
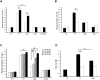
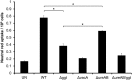
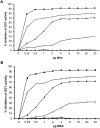
Helicobacter pylori causes cellular vacuolation in host cells, a cytotoxic event attributed to vacuolating cytotoxin (VacA) and the presence of permeant weak bases such as ammonia. We report here the role of γ-glutamyl transpeptidase (GGT), a constitutively expressed secretory enzyme of H. pylori, in potentiating VacA-dependent vacuolation formation in H. pylori-infected AGS and primary gastric cells. The enhancement is brought about by GGT hydrolysing glutamine present in the extracellular medium, thereby releasing ammonia which accentuates the VacA-induced vacuolation. The events of vacuolation in H. pylori wild type (WT)- and Δggt-infected AGS cells were first captured and visualized by real-time phase-contrast microscopy where WT was observed to induce more vacuoles than Δggt. By using semi-quantitative neutral red uptake assay, we next showed that Δggt induced significantly less vacuolation in AGS and primary gastric epithelial cells as compared to the parental strain (P<0.05) indicating that GGT potentiates the vacuolating effect of VacA. Notably, vacuolation induced by WT was significantly reduced in the absence of GGT substrate, glutamine (P<0.05) or in the presence of a competitive GGT inhibitor, serine-borate complex. Furthermore, the vacuolating ability of Δggt was markedly restored when co-incubated with purified recombinant GGT (rGGT), although rGGT itself did not induce vacuolation independently. Similarly, the addition of exogenous ammonium chloride as a source of ammonia also rescued the ability of Δggt to induce vacuolation. Additionally, we also show that monoclonal antibodies against GGT effectively inhibited GGT activity and successfully suppressed H. pylori-induced vacuolation. Collectively, our results clearly demonstrate that generation of ammonia by GGT through glutamine hydrolysis is responsible for enhancing VacA-dependent vacuolation. Our findings provide a new perspective on GGT as an important virulence factor and a promising target in the management of H. pylori-associated gastric diseases.
Conflict of interest statement
Figures

Similar articles
-
Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin.Cancer Res. 2003 Mar 1;63(5):951-7. Cancer Res. 2003. PMID: 12615708
-
Helicobacter pylori gamma-glutamyl transpeptidase is a pathogenic factor in the development of peptic ulcer disease.Gastroenterology. 2010 Aug;139(2):564-73. doi: 10.1053/j.gastro.2010.03.050. Epub 2010 Mar 27. Gastroenterology. 2010. PMID: 20347814
-
Significance of ammonia in the genesis of gastric epithelial lesions induced by Helicobacter pylori: an in vitro study with different bacterial strains and urea concentrations.Digestion. 1996;57(5):299-304. doi: 10.1159/000201349. Digestion. 1996. PMID: 8886572
-
Helicobacter pylori gamma-glutamyl transpeptidase and its pathogenic role.World J Gastroenterol. 2014 Jan 21;20(3):630-8. doi: 10.3748/wjg.v20.i3.630. World J Gastroenterol. 2014. PMID: 24574736 Free PMC article. Review.
-
Helicobacter pylori vacuolating cytotoxin, VacA, is responsible for gastric ulceration.J Biochem. 2004 Dec;136(6):741-6. doi: 10.1093/jb/mvh181. J Biochem. 2004. PMID: 15671482 Review.
Cited by
-
Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity.Toxins (Basel). 2019 Nov 19;11(11):677. doi: 10.3390/toxins11110677. Toxins (Basel). 2019. PMID: 31752394 Free PMC article. Review.
-
Construction and preservation of a stable and highly expressed recombinant Helicobacter pylori vacuolating cytotoxin A with apoptotic activity.BMC Microbiol. 2021 Aug 18;21(1):229. doi: 10.1186/s12866-021-02262-7. BMC Microbiol. 2021. PMID: 34407768 Free PMC article.
-
Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals.Int J Mol Sci. 2020 Sep 3;21(17):6451. doi: 10.3390/ijms21176451. Int J Mol Sci. 2020. PMID: 32899442 Free PMC article. Review.
-
Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment.Cells. 2020 Dec 25;10(1):27. doi: 10.3390/cells10010027. Cells. 2020. PMID: 33375694 Free PMC article. Review.
-
Intracellular Degradation of Helicobacter pylori VacA Toxin as a Determinant of Gastric Epithelial Cell Viability.Infect Immun. 2019 Mar 25;87(4):e00783-18. doi: 10.1128/IAI.00783-18. Print 2019 Apr. Infect Immun. 2019. PMID: 30692181 Free PMC article.
References
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1: 1311–1315. - PubMed
-
- Cover TL, Blaser MJ. Helicobacter pylori infection, a paradigm for chronic mucosal inflammation: pathogenesis and implications for eradication and prevention. Adv Intern Med. 1996;41: 85–117. - PubMed
-
- Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1: 63–96. - PubMed
-
- Chevalier C, Thiberge JM, Ferrero RL, Labigne A. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol. 1999;31: 1359–1372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

