Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging
- PMID: 26111777
- PMCID: PMC4568976
- DOI: 10.1111/acel.12368
Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging
Abstract
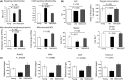
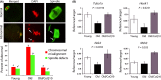
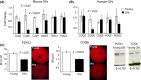
Female reproductive capacity declines dramatically in the fourth decade of life as a result of an age-related decrease in oocyte quality and quantity. The primary causes of reproductive aging and the molecular factors responsible for decreased oocyte quality remain elusive. Here, we show that aging of the female germ line is accompanied by mitochondrial dysfunction associated with decreased oxidative phosphorylation and reduced Adenosine tri-phosphate (ATP) level. Diminished expression of the enzymes responsible for CoQ production, Pdss2 and Coq6, was observed in oocytes of older females in both mouse and human. The age-related decline in oocyte quality and quantity could be reversed by the administration of CoQ10. Oocyte-specific disruption of Pdss2 recapitulated many of the mitochondrial and reproductive phenotypes observed in the old females including reduced ATP production and increased meiotic spindle abnormalities, resulting in infertility. Ovarian reserve in the oocyte-specific Pdss2-deficient animals was diminished, leading to premature ovarian failure which could be prevented by maternal dietary administration of CoQ10. We conclude that impaired mitochondrial performance created by suboptimal CoQ10 availability can drive age-associated oocyte deficits causing infertility.
Keywords: Mitochondria; anti-aging; fecundity; individual; molecular biology of aging; mouse models.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures


References
-
- Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum. Reprod. 1996;11:2217–2222. - PubMed
-
- Chi MM, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. Am. J. Physiol. Endocrinol. Metab. 2002;283:E226–E232. - PubMed
-
- Crane FL. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001;20:591–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

