Management of the respiratory distress symptom cluster in lung cancer: a randomised controlled feasibility trial
- PMID: 26111954
- PMCID: PMC4584102
- DOI: 10.1007/s00520-015-2810-x
Management of the respiratory distress symptom cluster in lung cancer: a randomised controlled feasibility trial
Abstract
Background: Breathlessness, cough and fatigue are distressing symptoms for patients with lung cancer. There is evidence that these three symptoms form a discreet symptom cluster. This study aimed to feasibly test a new non-pharmacological intervention for the management of the Respiratory Distress Symptom Cluster (breathlessness-cough-fatigue) in lung cancer.
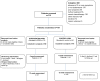
Method: This was a multi-centre, randomised controlled non-blinded parallel group feasibility trial. Eligible patients (patients with primary lung cancer and 'bothered' by at least two of the three cluster symptoms) received usual care plus a multicomponent intervention delivered over two intervention training sessions and a follow-up telephone call or usual care only. Follow-up was for 12 weeks, and end-points included six numerical rating scales for breathlessness severity, Dyspnoea-12, Manchester Cough in Lung Cancer scale, FACIT-Fatigue scale, Hospital Anxiety and Depression scale, Lung Cancer Symptom Scale and the EQ-5D-3L, collected at baseline, week 4 and week 12.
Results: One hundred seven patients were randomised over 8 months; however, six were removed from further analysis due to protocol violations (intervention group n = 50 and control group n = 51). Of the ineligible patients (n = 608), 29 % reported either not experiencing two or more symptoms or not being 'bothered' by at least two symptoms. There was 29 % drop-out by week 4, and by week 12, a further two patients in the control group were lost to follow-up. A sample size calculation indicated that 122 patients per arm would be needed to detect a clinically important difference in the main outcome for breathlessness, cough and fatigue.
Conclusions: The study has provided evidence of the feasibility and acceptability of a new intervention in the lung cancer population and warrants a fully powered trial before we reach any conclusions. The follow-on trial will test the hypothesis that the intervention improves symptom cluster of breathlessness, cough and fatigue better than usual care alone. Full economic evaluation will be conducted in the main trial.
Keywords: Breathlessness; Cough; Dyspnoea; Fatigue; Lung cancer; Non-pharmacological intervention; Respiratory symptoms; Self-management; Symptom cluster; Symptoms.
Figures

References
-
- Cheville AL, Novotny PJ, Sloan JA, Basford JR, Wampfler JA, Garces YI, Jatoi A, Yang P. Fatigue, dyspnea, and cough comprise a persistent symptom cluster up to five years after diagnosis with lung cancer. J Pain Symptom Manag. 2011;42(2):202–12. doi: 10.1016/j.jpainsymman.2010.10.257. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

