Li[Li0.2Ni 0.16Mn 0.56Co 0.08]O 2 Nanoparticle/Carbon Composite Using Polydopamine Binding Agent for Enhanced Electrochemical Performance
- PMID: 26111979
- PMCID: PMC4481243
- DOI: 10.1186/s11671-015-0986-0
Li[Li0.2Ni 0.16Mn 0.56Co 0.08]O 2 Nanoparticle/Carbon Composite Using Polydopamine Binding Agent for Enhanced Electrochemical Performance
Abstract
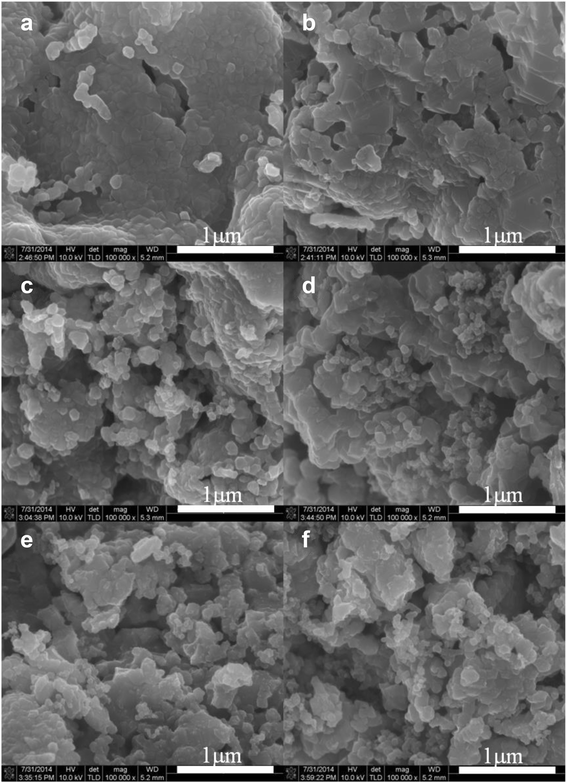
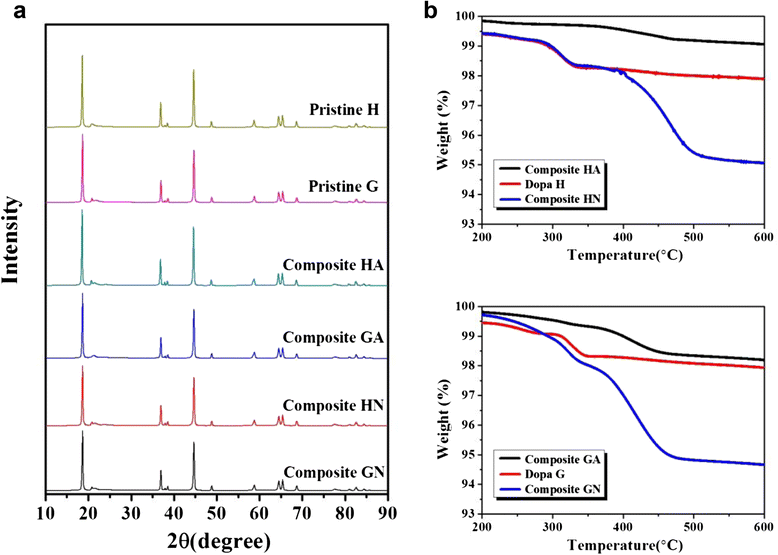
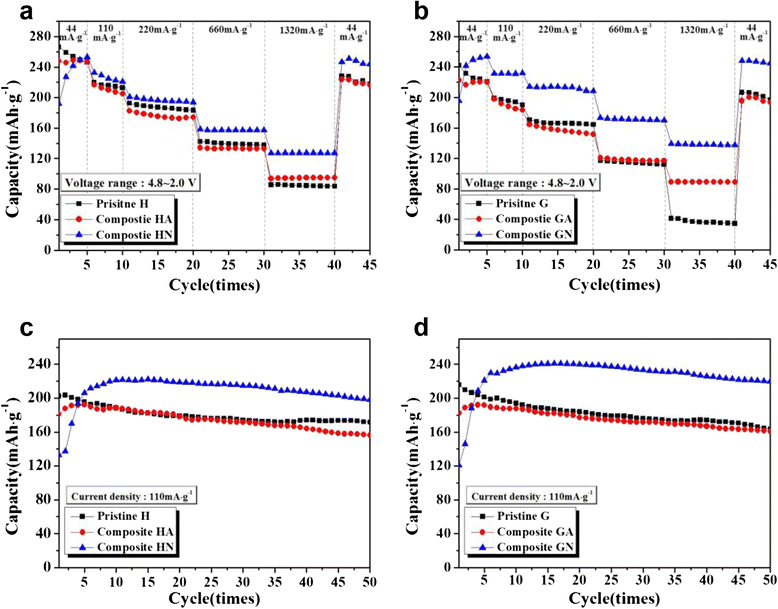
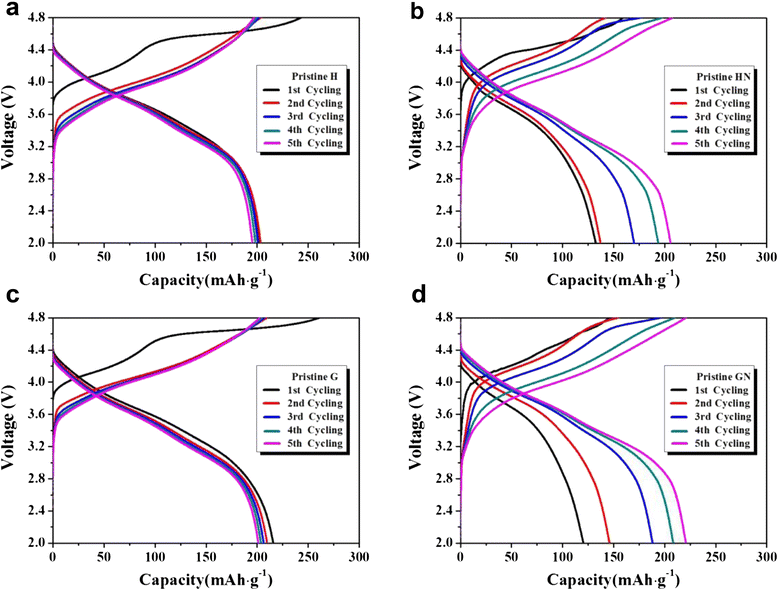
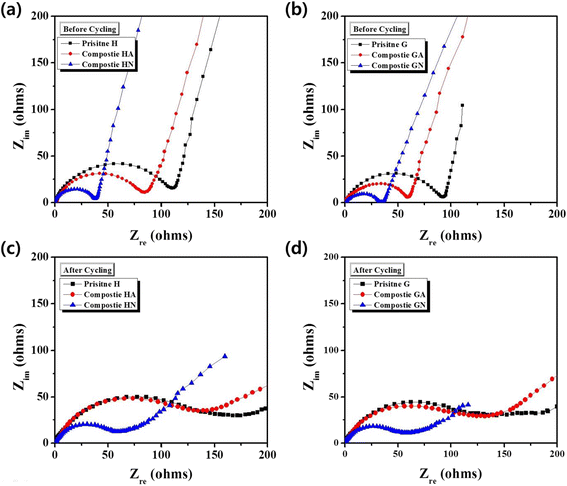
Li[Li0.2Ni0.16Mn0.56Co0.08]O2 nanoparticles were composited with carbon (Super P) in order to achieve an enhanced rate capability. A polydopamine pre-coating layer was introduced to facilitate the adhesion between Super P and pristine nanoparticles. The Super P particles were dispersed on the surface of Li[Li0.2Ni0.16Mn0.56Co0.08]O2 powders. The composite samples that were heat-treated in a N2 atmosphere showed increased capacity and enhanced rate capability, which was caused by the improved electronic conductivity owing to the presence of carbon. However, the composite samples that were heat-treated in air did not present these carbon-related effects clearly. The capacity changes observed during the first several cycles may be due to the oxygen deficiency of the structure caused by the heat-treatment process.
Figures

Similar articles
-
Characterization and Control of Irreversible Reaction in Li-Rich Cathode during the Initial Charge Process.ACS Appl Mater Interfaces. 2018 Apr 4;10(13):10804-10818. doi: 10.1021/acsami.7b12722. Epub 2018 Mar 21. ACS Appl Mater Interfaces. 2018. PMID: 29561131
-
Impact of surface coating on electrochemical and thermal behaviors of a Li-rich Li1.2Ni0.16Mn0.56Co0.08O2 cathode.RSC Adv. 2020 Apr 17;10(26):15274-15281. doi: 10.1039/d0ra02060e. eCollection 2020 Apr 16. RSC Adv. 2020. PMID: 35495434 Free PMC article.
-
Flexible Li[Li0.2Ni0.13Co0.13Mn0.54]O2/Carbon Nanotubes/Nanofibrillated Celluloses Composite Electrode for High-Performance Lithium-Ion Battery.Front Chem. 2019 Aug 6;7:555. doi: 10.3389/fchem.2019.00555. eCollection 2019. Front Chem. 2019. PMID: 31448262 Free PMC article.
-
Multifunctional AlPO4 coating for improving electrochemical properties of low-cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials for lithium-ion batteries.ACS Appl Mater Interfaces. 2015 Feb 18;7(6):3773-81. doi: 10.1021/am508579r. Epub 2015 Feb 5. ACS Appl Mater Interfaces. 2015. PMID: 25629768
-
Integration of inorganic nanostructures with polydopamine-derived carbon: tunable morphologies and versatile applications.Nanoscale. 2016 Jan 28;8(4):1770-88. doi: 10.1039/c5nr06711a. Nanoscale. 2016. PMID: 26750427 Review.
References
-
- Kim CS, Cho JH, Park YJ. Li[Li0.2Ni0.16Mn0.56Co0.08]O2 nanoparticles prepared using a surfactant modified combustion method. J Electroceram. 2014;32:324–31. doi: 10.1007/s10832-014-9905-5. - DOI
-
- Song HG, Kim JY, Kim KT, Park YJ. Enhanced electrochemical properties of Li(Ni0.4Co0.3Mn0.3)O2 cathode by surface modification using Li3PO4-based materials. J Power Sources. 2011;196:6847–55. doi: 10.1016/j.jpowsour.2010.09.027. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

