Glycan complexity dictates microbial resource allocation in the large intestine
- PMID: 26112186
- PMCID: PMC4491172
- DOI: 10.1038/ncomms8481
Glycan complexity dictates microbial resource allocation in the large intestine
Erratum in
-
Corrigendum: Glycan complexity dictates microbial resource allocation in the large intestine.Nat Commun. 2016 Feb 5;7:10705. doi: 10.1038/ncomms10705. Nat Commun. 2016. PMID: 26848059 Free PMC article. No abstract available.
Abstract
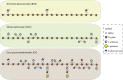
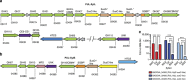
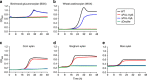
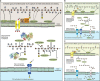
The structure of the human gut microbiota is controlled primarily through the degradation of complex dietary carbohydrates, but the extent to which carbohydrate breakdown products are shared between members of the microbiota is unclear. We show here, using xylan as a model, that sharing the breakdown products of complex carbohydrates by key members of the microbiota, such as Bacteroides ovatus, is dependent on the complexity of the target glycan. Characterization of the extensive xylan degrading apparatus expressed by B. ovatus reveals that the breakdown of the polysaccharide by the human gut microbiota is significantly more complex than previous models suggested, which were based on the deconstruction of xylans containing limited monosaccharide side chains. Our report presents a highly complex and dynamic xylan degrading apparatus that is fine-tuned to recognize the different forms of the polysaccharide presented to the human gut microbiota.
Figures




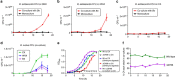
References
-
- Backhed F., Ley R. E., Sonnenburg J. L., Peterson D. A. & Gordon J. I. Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F014163/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G016186/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G016240/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 097907/WT_/Wellcome Trust/United Kingdom
- WT097907AIA/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

