Co-translational capturing of nascent ribosomal proteins by their dedicated chaperones
- PMID: 26112308
- PMCID: PMC4491177
- DOI: 10.1038/ncomms8494
Co-translational capturing of nascent ribosomal proteins by their dedicated chaperones
Abstract
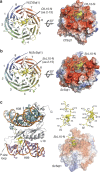
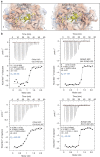
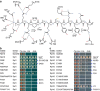
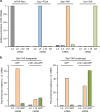
Exponentially growing yeast cells produce every minute >160,000 ribosomal proteins. Owing to their difficult physicochemical properties, the synthesis of assembly-competent ribosomal proteins represents a major challenge. Recent evidence highlights that dedicated chaperone proteins recognize the N-terminal regions of ribosomal proteins and promote their soluble expression and delivery to the assembly site. Here we explore the intuitive possibility that ribosomal proteins are captured by dedicated chaperones in a co-translational manner. Affinity purification of four chaperones (Rrb1, Syo1, Sqt1 and Yar1) selectively enriched the mRNAs encoding their specific ribosomal protein clients (Rpl3, Rpl5, Rpl10 and Rps3). X-ray crystallography reveals how the N-terminal, rRNA-binding residues of Rpl10 are shielded by Sqt1's WD-repeat β-propeller, providing mechanistic insight into the incorporation of Rpl10 into pre-60S subunits. Co-translational capturing of nascent ribosomal proteins by dedicated chaperones constitutes an elegant mechanism to prevent unspecific interactions and aggregation of ribosomal proteins on their road to incorporation.
Figures




Similar articles
-
Dedicated chaperones coordinate co-translational regulation of ribosomal protein production with ribosome assembly to preserve proteostasis.Elife. 2022 Mar 31;11:e74255. doi: 10.7554/eLife.74255. Elife. 2022. PMID: 35357307 Free PMC article.
-
Crystal structure of the yeast ribosomal protein rpS3 in complex with its chaperone Yar1.J Mol Biol. 2013 Nov 15;425(22):4154-60. doi: 10.1016/j.jmb.2013.08.022. Epub 2013 Sep 7. J Mol Biol. 2013. PMID: 24021814
-
Synchronizing nuclear import of ribosomal proteins with ribosome assembly.Science. 2012 Nov 2;338(6107):666-71. doi: 10.1126/science.1226960. Science. 2012. PMID: 23118189
-
Nuclear import of dimerized ribosomal protein Rps3 in complex with its chaperone Yar1.Sci Rep. 2016 Nov 7;6:36714. doi: 10.1038/srep36714. Sci Rep. 2016. PMID: 27819319 Free PMC article.
-
Hold on to your friends: Dedicated chaperones of ribosomal proteins: Dedicated chaperones mediate the safe transfer of ribosomal proteins to their site of pre-ribosome incorporation.Bioessays. 2017 Jan;39(1):1-12. doi: 10.1002/bies.201600153. Epub 2016 Nov 17. Bioessays. 2017. PMID: 27859409 Review.
Cited by
-
Tsr4 and Nap1, two novel members of the ribosomal protein chaperOME.Nucleic Acids Res. 2019 Jul 26;47(13):6984-7002. doi: 10.1093/nar/gkz317. Nucleic Acids Res. 2019. PMID: 31062022 Free PMC article.
-
Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins.Int J Mol Sci. 2021 May 23;22(11):5496. doi: 10.3390/ijms22115496. Int J Mol Sci. 2021. PMID: 34071057 Free PMC article. Review.
-
The eukaryote-specific N-terminal extension of ribosomal protein S31 contributes to the assembly and function of 40S ribosomal subunits.Nucleic Acids Res. 2016 Sep 19;44(16):7777-91. doi: 10.1093/nar/gkw641. Epub 2016 Jul 15. Nucleic Acids Res. 2016. PMID: 27422873 Free PMC article.
-
Structural basis for (p)ppGpp-mediated inhibition of the GTPase RbgA.J Biol Chem. 2018 Dec 21;293(51):19699-19709. doi: 10.1074/jbc.RA118.003070. Epub 2018 Oct 26. J Biol Chem. 2018. PMID: 30366986 Free PMC article.
-
Molecular basis for protection of ribosomal protein L4 from cellular degradation.Nat Commun. 2017 Feb 2;8:14354. doi: 10.1038/ncomms14354. Nat Commun. 2017. PMID: 28148929 Free PMC article.
References
-
- Ben-Shem A. et al. The structure of the eukaryotic ribosome at 3.0Å resolution. Science 334, 1524–1529 (2011). - PubMed
-
- Klinge S., Voigts-Hoffmann F., Leibundgut M., Arpagaus S. & Ban N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334, 941–948 (2011). - PubMed
-
- Melnikov S. et al. One core, two shells: bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol. 19, 560–567 (2012). - PubMed
-
- Rabl J., Leibundgut M., Ataide S. F., Haag A. & Ban N. Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1. Science 331, 730–736 (2011). - PubMed
-
- Warner J. R. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24, 437–440 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

