Hormone naïve prostate cancer: predicting and maximizing response intervals
- PMID: 26112479
- PMCID: PMC4814946
- DOI: 10.4103/1008-682X.152821
Hormone naïve prostate cancer: predicting and maximizing response intervals
Abstract
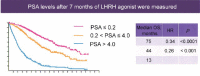
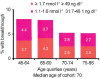
Hormone naïve advanced prostate cancer is subdivided into two disease states: biochemical recurrence and traditional M1 (metastatic) prostate cancer and characterized by no prior hormonal therapy or androgen deprivation therapy (ADT). In biochemical recurrence/prostate-specific antigen (PSA) recurrence, men should be risk-stratified based on their PSA doubling time, the Gleason score and the timing of the recurrence. In general, only men who are at high risk should be considered for early/immediate ADT although this is best done using shared decision with the patient. The type of ADT to be used in biochemical recurrence ranging from oral-only peripheral blockade (peripheral androgen deprivation) to complete hormonal therapy (combined androgen blockade [CAB]) remains in debate owing to lack of randomized controlled trials (RCT). However, there is good RCT support for use of intermittent hormonal therapy (IHT). There is also limited research on biomarker response (PSA and testosterone decline) to predict prognosis. On the other hand, in the setting of M1 hormone naïve prostate cancer, there are many more RCT's to inform our decisions. CAB and gonadotrophin-releasing hormone antagonists perhaps provide a slight efficacy advantage while IHT may be slightly inferior with minimal M1 disease. The PSA nadir at 7 months after starting ADT is a powerful prognostic tool for M1 patients. There is growing recognition that serum testosterone (T) control while on ADT is linked to the development of castrate-resistant prostate cancer. Especially for a M1 patient, maintaining a serum T below 20-30 ng dl-1 prolongs the response to ADT. Novel oral agents (abiraterone and enzalutamide) may soon find use in hormone naïve disease and may alter the treatment landscape. Despite over 75 years of experience with ADT, many questions remain, and the field continues to evolve.
Figures


References
-
- Moul JW, Kibel AS, Roach M, 3rd, Dreicer R. Indications and practice with androgen deprivation therapy. Urology. 2011;78:S478–81. - PubMed
-
- Moul JW, Evans CP, Gomella LG, Roach M, 3rd, Dreicer R. Traditional approaches to androgen deprivation therapy. Urology. 2011;78:S485–93. - PubMed
-
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate 1941. J Urol. 2002;168:9–12. - PubMed
-
- Bailar JC, 3rd, Byar DP. Estrogen treatment for cancer of the prostate. Early results with 3 doses of diethylstilbestrol and placebo. Cancer. 1970;26:257–61. - PubMed
-
- Byar DP. Proceedings: the Veterans Administration Cooperative Urological Research Group's studies of cancer of the prostate. Cancer. 1973;32:1126–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

