Chronic hepatitis B: A wave of new therapies on the horizon
- PMID: 26112647
- PMCID: PMC5391532
- DOI: 10.1016/j.antiviral.2015.06.014
Chronic hepatitis B: A wave of new therapies on the horizon
Abstract
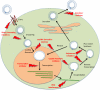
This year marks the 50th anniversary of the discovery of the Australia antigen (Blumberg et al., 1965), which in 1967 was identified to be the hepatitis B virus (HBV) surface antigen. Even though several antiviral medications have been in use for the management of chronic HBV infection for more than 20years, sustained clearance of HBsAg, similar to the sustained viral response (SVR) or cure in chronic hepatitis C, occurs in only a minority of treated patients. Moreover, even after 10years of effective suppression of HBV viremia with current therapy, there is only a 40-70% reduction in deaths from liver cancer. Recent success in developing antivirals for hepatitis C that are effective across all genotypes has renewed interest in a similar cure for chronic HBV infection. In this article, we review a wave of newly identified drug targets, investigational compounds and experimental strategies that are now under clinical evaluation or in preclinical development. The paper forms part of a symposium in Antiviral Research on "An unfinished story: From the discovery of the Australia antigen to the development of new curative therapies for hepatitis B."
Keywords: Antiviral therapy; Chronic hepatitis B; Clinical trials; Hepatitis B virus.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures




References
-
- Adam BL, Qu Y, Davis JW, Ward MD, Clements MA, Cazares LH, Semmes OJ, Schellhammer PF, Yasui Y, Feng Z, Wright GL., Jr. Serum protein fingerprinting coupled with a pattern-matching algorithm distinguishes prostate cancer from benign prostate hyperplasia and healthy men. Cancer research. 2002;62:3609–3614. - PubMed
-
- Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. The New England journal of medicine. 2014;370:1483–1493. - PubMed
-
- Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, Mutimer D, Deterding K, Reijnders JG, Oo Y, Petersen J, van Bommel F, de Knegt RJ, Santantonio T, Berg T, Welzel TM, Wedemeyer H, Buti M, Pradat P, Zoulim F, Hansen B, Janssen HL. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. 2014 - PubMed
-
- Arrowhead Arrowhead Provides Update on IND for ARC-520 Phase 2b Study. 2015b.
-
- Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond) 2011;61:531–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

