Kinetics and fracture resistance of lithiated silicon nanostructure pairs controlled by their mechanical interaction
- PMID: 26112834
- PMCID: PMC4491816
- DOI: 10.1038/ncomms8533
Kinetics and fracture resistance of lithiated silicon nanostructure pairs controlled by their mechanical interaction
Abstract
Following an explosion of studies of silicon as a negative electrode for Li-ion batteries, the anomalous volumetric changes and fracture of lithiated single Si particles have attracted significant attention in various fields, including mechanics. However, in real batteries, lithiation occurs simultaneously in clusters of Si in a confined medium. Hence, understanding how the individual Si structures interact during lithiation in a closed space is necessary. Here, we demonstrate physical and mechanical interactions of swelling Si structures during lithiation using well-defined Si nanopillar pairs. Ex situ SEM and in situ TEM studies reveal that compressive stresses change the reaction kinetics so that preferential lithiation occurs at free surfaces when the pillars are mechanically clamped. Such mechanical interactions enhance the fracture resistance of lithiated Si by lessening the tensile stress concentrations in Si structures. This study will contribute to improved design of Si structures at the electrode level for high-performance Li-ion batteries.
Figures
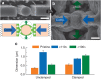

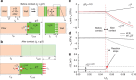
 ). Red vertical lines indicate the contact and reaction stoppage on lithiation of Si, respectively.
). Red vertical lines indicate the contact and reaction stoppage on lithiation of Si, respectively.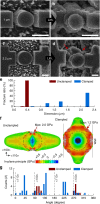
References
-
- Chan C. K. et al. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotech. 3, 31–35 (2008). - PubMed
-
- Wu H. & Cui Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 7, 414–429 (2012).
-
- Whittingham M. S. Materials challenges facing electrical energy storage. MRS Bull. 33, 411–421 (2008).
-
- McDowell M. T., Lee S. W., Nix W. D. & Cui Y. Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 25, 4966–4985 (2013). - PubMed
-
- Kasavajjula U., Wang C. & Appleby a. J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 163, 1003–1039 (2007).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

