Tumor necrosis factor-α enhances voltage-gated Na⁺ currents in primary culture of mouse cortical neurons
- PMID: 26112872
- PMCID: PMC4510892
- DOI: 10.1186/s12974-015-0349-x
Tumor necrosis factor-α enhances voltage-gated Na⁺ currents in primary culture of mouse cortical neurons
Abstract
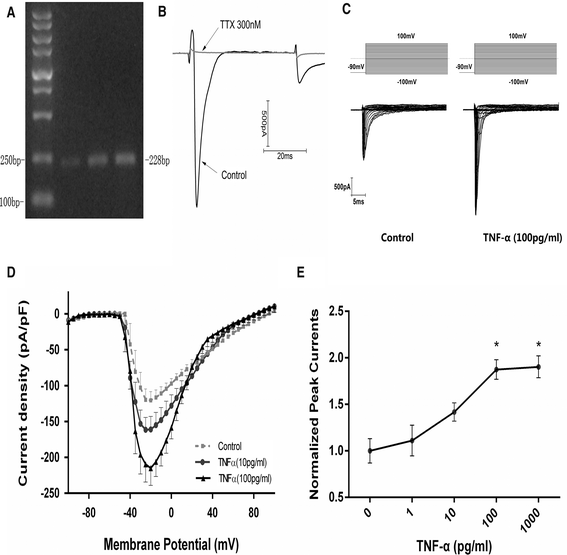
Background: Previous studies showed that TNF-α could activate voltage-gated Na(+) channels (VGSCs) in the peripheral nervous system (PNS). Since TNF-α is implicated in many central nervous system (CNS) diseases, we examined potential effects of TNF-α on VGSCs in the CNS.
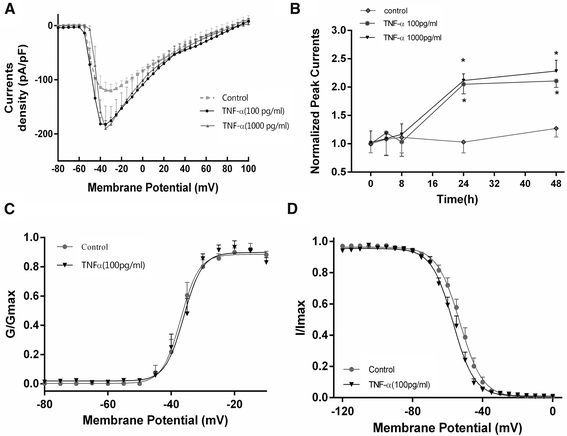
Methods: Effects of TNF-α (1-1000 pg/mL, for 4-48 h) on VGSC currents were examined using whole-cell voltage clamp and current clamp techniques in primary culture of mouse cortical neurons. Expression of Nav1.1, Nav1.2, Nav1.3, and Nav1.6 were examined at both the mRNA and protein levels, prior to and after TNF-α exposure.
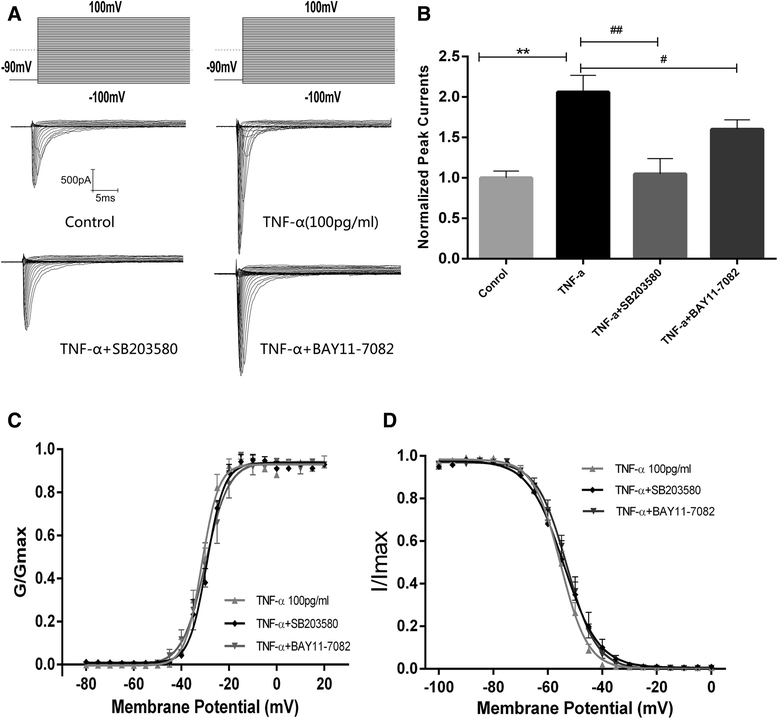
Results: TNF-α increased Na(+) currents by accelerating the activation of VGSCs. The threshold for action potential (AP) was decreased and firing rate were increased. VGSCs were up-regulated at both the mRNA and protein levels. The observed effects of TNF-α on Na(+) currents were inhibited by pre-incubation with the NF-κB inhibitor BAY 11-7082 (1 μM) or the p38 mitogen-activated protein kinases (MAPK) inhibitor SB203580 (1 μM).
Conclusions: TNF-α increases Na(+) currents by accelerating the channel activation as well as increasing the expression of VGSCs in a mechanism dependent upon NF-κB and p38 MAPK signal pathways in CNS neurons.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

