Characterisation of Dermanyssus gallinae glutathione S-transferases and their potential as acaricide detoxification proteins
- PMID: 26112960
- PMCID: PMC4491418
- DOI: 10.1186/s13071-015-0960-9
Characterisation of Dermanyssus gallinae glutathione S-transferases and their potential as acaricide detoxification proteins
Abstract
Background: Glutathione S-transferases (GSTs) facilitate detoxification of drugs by catalysing the conjugation of the reduced glutathione (GSH) to electrophilic xenobiotic substrates and therefore have a function in multi-drug resistance. As a result, knowledge of GSTs can inform both drug resistance in, and novel interventions for, the control of endo- and ectoparasite species. Acaricide resistance and the need for novel control methods are both pressing needs for Dermanyssus gallinae, a highly economically important haematophagous ectoparasite of poultry.
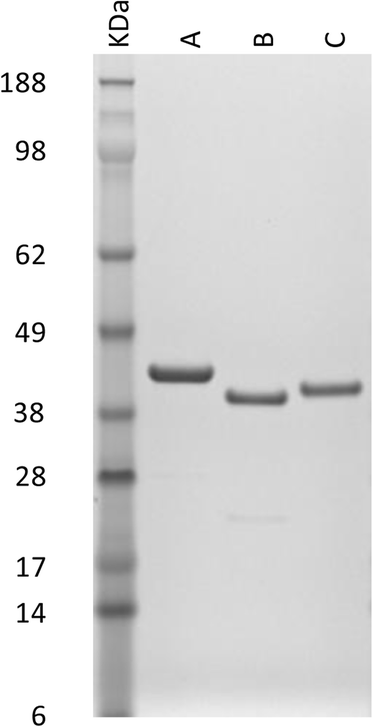
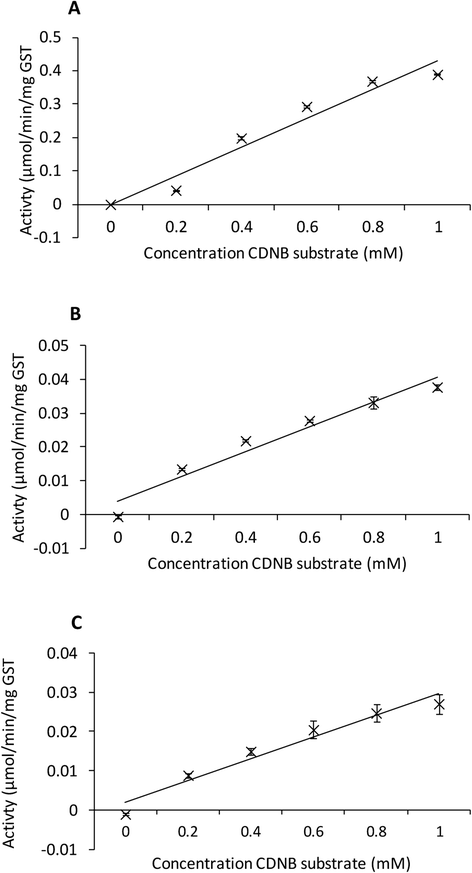
Methods: A transcriptomic database representing D. gallinae was examined and 11 contig sequences were identified with GST BlastX identities. The transcripts represented by 3 contigs, designated Deg-GST-1, -2 and -3, were fully sequenced and further characterized by phylogenetic analysis. Recombinant versions of Deg-GST-1, -2 and -3 (rDeg-GST) were enzymically active and acaricide-binding properties of the rDeg-GSTs were established by evaluating the ability of selected acaricides to inhibit the enzymatic activity of rDeg-GSTs.
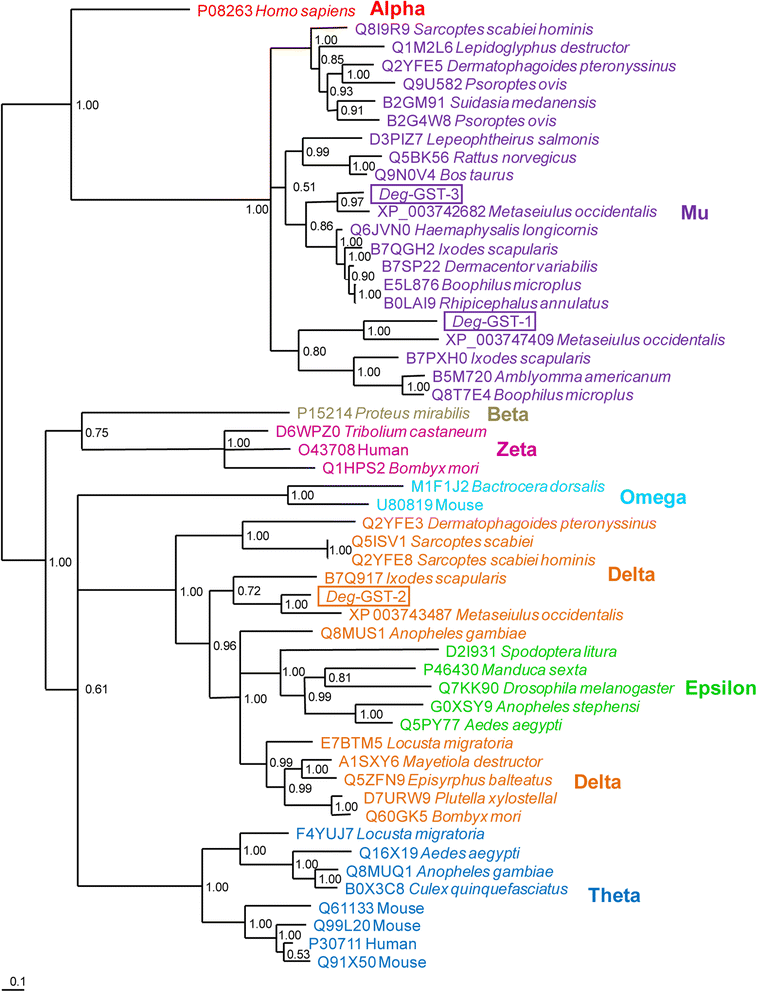
Results: 6 of the identified GSTs belonged to the mu class, followed by 3 kappa, 1 omega and 1 delta class molecules. Deg-GST-1 and -3 clearly partitioned with orthologous mu class GSTs and Deg-GST-2 partitioned with delta class GSTs. Phoxim, permethrin and abamectin significantly inhibited rDeg-GST-1 activity by 56, 35 and 17% respectively. Phoxim also inhibited rDeg-2-GST (14.8%) and rDeg-GST-3 (20.6%) activities.
Conclusions: Deg-GSTs may have important roles in the detoxification of pesticides and, with the increased occurrence of acaricide resistance in this species worldwide, Deg-GSTs are attractive targets for novel interventions.
Figures

References
-
- Van Emous R. Wage war against the red mite! Poultry Int. 2005;4:26–33.
-
- Sparagano O, Pavlicevic A, Murano T, Camarda A, Sahibi H, Kilpinen O, Mul M, van Emous R, le Bouquin S, Hoel K, Cafiero MA. Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems. Exp Appl Acarol. 2009;48:3–10. doi: 10.1007/s10493-008-9233-z. - DOI - PubMed
-
- Mul FM. Control of Poultry Red Mite in layer farms using an automated monitoring device Prevalence and effects of Dermanyssus gallinae. In: van Neikerk GCMT, Meerburg GB, Groot-Keorkamp WGP, editors. XVIIIth World Veterinary Poultry Association Congress Proceedings. 2013; France.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

